Answered step by step
Verified Expert Solution
Question
1 Approved Answer
General Test V-8. 4 2 1. Write the equations of the reactions. Write the electron balance and put the coefficients and point out the oxidant
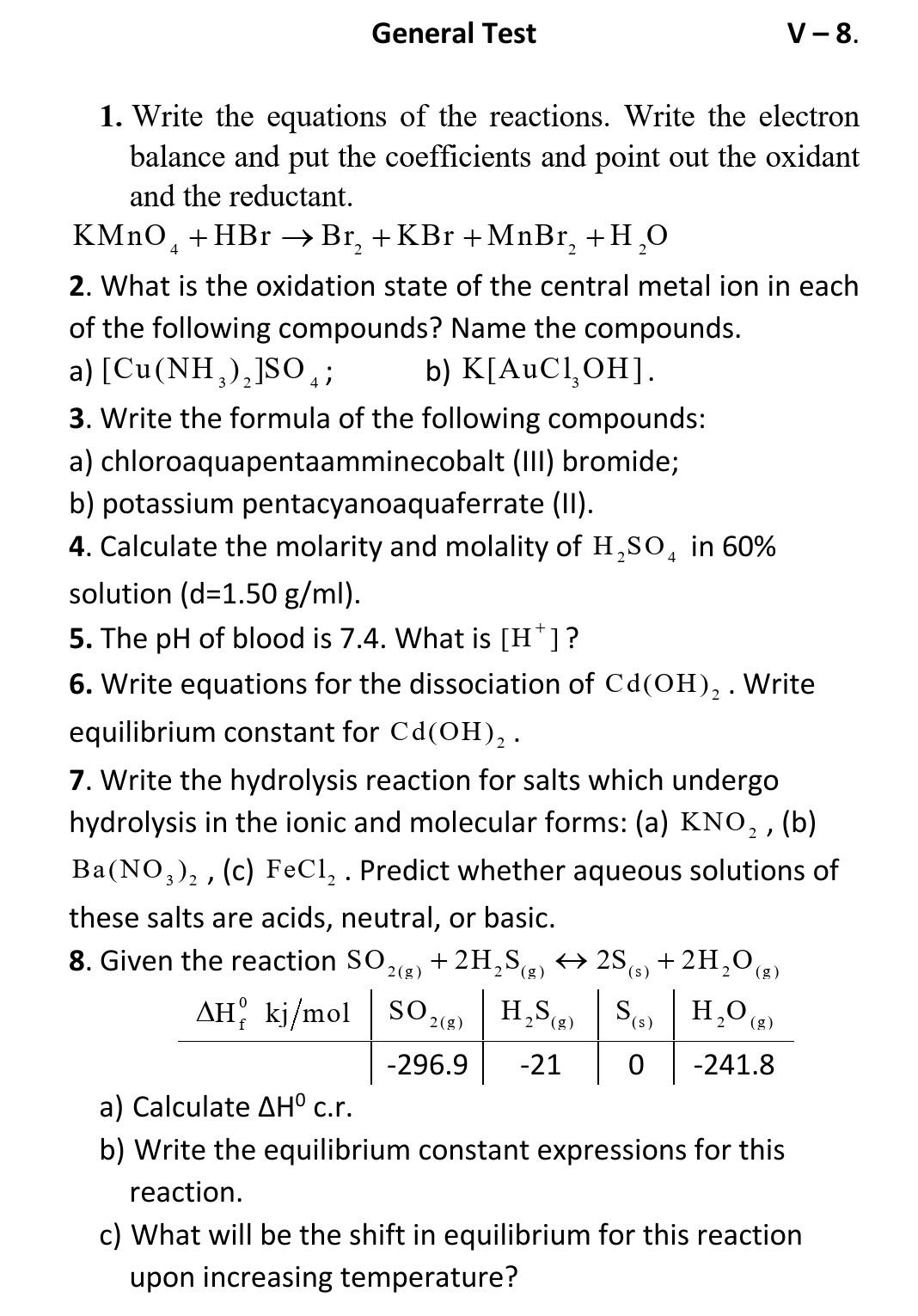
General Test V-8. 4 2 1. Write the equations of the reactions. Write the electron balance and put the coefficients and point out the oxidant and the reductant. KMnO, +HBr Br, +KBr +MnBr, +H,O 2. What is the oxidation state of the central metal ion in each of the following compounds? Name the compounds. a) [Cu(NH2),]SO 4; b) K[AuCl,OH]. 3. Write the formula of the following compounds: a) chloroaquapentaamminecobalt (III) bromide; b) potassium pentacyanoaquaferrate (II). 4. Calculate the molarity and molality of H,SO4 in 60% solution (d=1.50 g/ml). 5. The pH of blood is 7.4. What is [H]? 6. Write equations for the dissociation of Cd(OH)2. Write equilibrium constant for Cd(OH)2. 7. Write the hydrolysis reaction for salts which undergo hydrolysis in the ionic and molecular forms: (a) KNO, , (b) Ba(NO3)2 , (c) FeCl, . Predict whether aqueous solutions of these salts are acids, neutral, or basic. 8. Given the reaction SO2(g) + 2H, Sg) + 2S3) + 2H2O(g) AH, kj/mol SO2(g) H,SE) SSH,O (2) -296.9 -21 -241.8 a) Calculate AH c.r. b) Write the equilibrium constant expressions for this reaction. c) What will be the shift in equilibrium for this reaction upon increasing temperature? . + ) ( (g 2 (g () 2 ||
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


