Question
Given that the concentration of hydronium ions in a solution with pH of 2 is 0.19%, what would be the concentration of hydronium ions

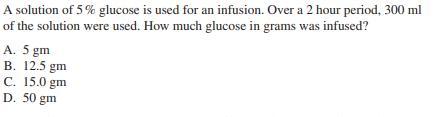
Given that the concentration of hydronium ions in a solution with pH of 2 is 0.19%, what would be the concentration of hydronium ions in a solution with pH of 3? A. 0.13% B. 0.29% C. 0.019% D. 1.9% A solution of 5% glucose is used for an infusion. Over a 2 hour period, 300 ml of the solution were used. How much glucose in grams was infused? A. 5 gm B. 12.5 gm C. 15.0 gm D. 50 gm
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below Part 1 Hydronium ion concentration Firs...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles The Quest For Insight
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
7th Edition
1464183953, 9781464183959
Students also viewed these Medical Sciences questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



