Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Given the following Thermodynamic Quantities at 298.15 K (25 C): Substance AH: (kJ/mol) S (J/K-mol) Cl2 (8) 222.96 P2 (g) PCI3 (g) 144.3 218.1
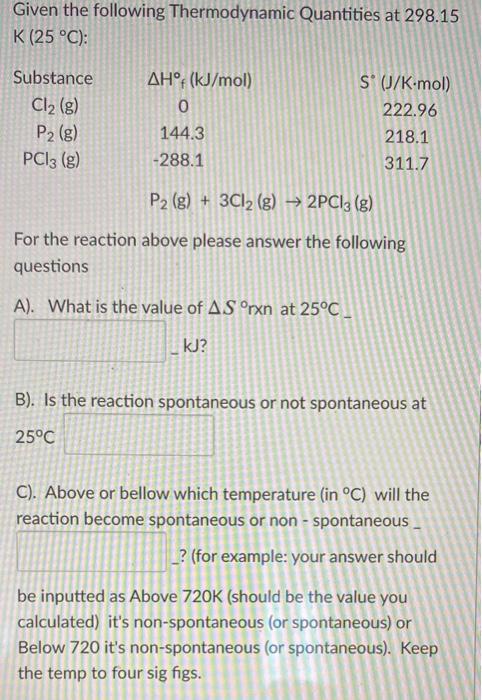
Given the following Thermodynamic Quantities at 298.15 K (25 C): Substance AH: (kJ/mol) S (J/K-mol) Cl2 (8) 222.96 P2 (g) PCI3 (g) 144.3 218.1 -288.1 311.7 P2 (g) + 3CI2 (g) 2PCI3 (g) For the reaction above please answer the following questions A). What is the value of AS rxn at 25C _ kJ? B). Is the reaction spontaneous or not spontaneous at 25C C). Above or bellow which temperature (in C) will the reaction become spontaneous or non - spontaneous _ ? (for example: your answer should be inputted as Above 720K (should be the value you calculated) it's non-spontaneous (or spontaneous) or Below 720 it's non-spontaneous (or spontaneous). Keep the temp to four sig figs.
Step by Step Solution
★★★★★
3.44 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
63643765a19ee_236387.pdf
180 KBs PDF File
63643765a19ee_236387.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


