Answered step by step
Verified Expert Solution
Question
1 Approved Answer
DIRECTIONS: 1. There are two parts to this group activity: a) Symbol and Calculated Atomic Mass b) Symbol and #p, #e, #n, A. 2
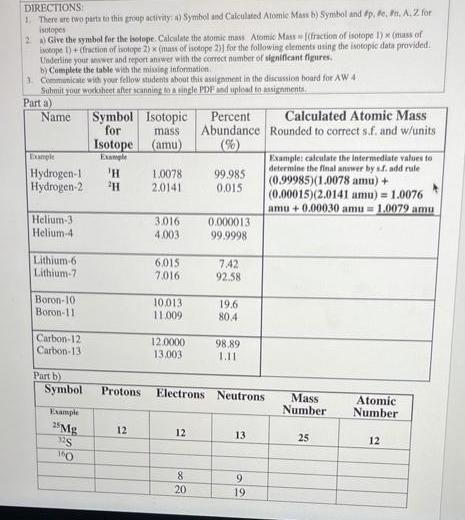
DIRECTIONS: 1. There are two parts to this group activity: a) Symbol and Calculated Atomic Mass b) Symbol and #p, #e, #n, A. 2 for isotopes 2) Give the symbol for the isotope. Calculate the atomic mass Atomic Mass [(fraction of isotope 1) x (mass of isotope 1)+ (fraction of isotope 2) x (mass of isotope 2)] for the following elements using the isotopic data provided. Underline your answer and report answer with the correct number of significant figures. b) Complete the table with the missing information. 3. Communicate with your fellow students about this assignment in the discussion board for AW 4 Submit your worksheet after scanning to a single PDF and upload to assignments. Parta) Name Helium-3 Helium-4 Example Hydrogen-1 'H Hydrogen-2 H Lithium-6. Lithium-7 Boron-10: Boron-11 Carbon-12 Carbon-13 Part b) Symbol Symbol for Example 25 Mg 32S 160 Isotope Example Protons 12 Isotopic mass. (amu) 1.0078 2.0141 3.016 4.003 6.015 7.016 10.013. 11.009 12.0000 13,003 12 Percent Abundance (%) 8 20 99.985 0.015 0.000013 99.9998 7.42 92.58 19.6 80.4 Electrons Neutrons 98,89 1.11 13 9 56 19 Calculated Atomic Mass Rounded to correct s.f. and w/units Example: calculate the intermediate values to determine the final answer by sf. add rule (0.99985)(1.0078 amu) + (0.00015)(2.0141 amu) = 1.0076 amu+0.00030 amu= 1.0079 amu Mass Number 25 Atomic Number 12
Step by Step Solution
★★★★★
3.30 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
Partla Name Symbol Isotopic Percent mass amu Abundance 1 H Hydrogin1 Hydrogen2 2H ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


