help asap

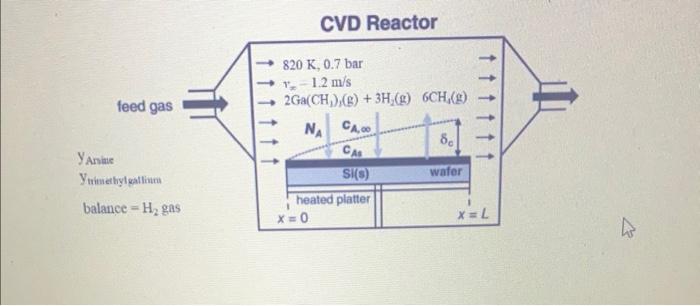
A horizontal chemical vapor deposition (CVD) reactor similar to the configuration shown in Example 3, Figure 28.6 will be used for growth of gallium arsenide (GaAs) thin films. In this process, arsine vapor, trimethylgallium vapor, and H2 gas are fed into the reactor. Inside the reactor, the silicon wafer rests on a heated plate called a susceptor. The plate and wafter are 15cm wide (into page) and 20cm long ( L in figure). The reactant gases flow parallel to the surface of the wafer and deposit a GaAs thin film according to the simplified CVD reactions 2AsH3(g)2As(s)+3H2(g)2Ga(CH3)3(g)+3H2(g)2Ga(s)+6CH4(g) If the reactant gas is considerably diluted in H2 gas, then the mass transfer of each species in the H2 carrier gas can be treated separately. These surface reactions are considered to be very rapid, and so the mass transfer of the gaseous reactants to the surface of the wafer limits the rate of GaAs thin-film formation. In the present process, a rectangular silicon wafer is positioned at the leading edge of the susceptor plate. The process temperature is 820K, and the total system pressure is 0.7 bar. The feed gas delivered to the reactor results in a bulk linear velocity of 1.2m/s. The arsine and trimethylgallium mole fractions in the feed gas are equal and both are small enough to be considered dilute. You may assume that the amount of arsine and trimethylgallium delivered with the feed gas is much higher than the amount of arsine and trimethylgallium consumed by the reactions, so that the concentration of these reactants in the bulk gas phase is essentially constant down the length of the reactor. You may also assume that the surface-reaction rates are instantaneous relative to the rates of mass transfer, so that the gas-phase concentrations of both arsine vapor and trimethylgallium vapor at the surface of the wafer are essentially zero. The binary gas-phase diffusion coefficient of trimethylgallium in H2 is 2.43cm2/s at 820K and 0.7 bar. You would like to deposit 1.0101gmoles of trimethylgallium onto the 1520cm wafer over a 5 minute period. What mole fraction of trimethylgallium must be in the H2 feed gas to achieve that? The figure below is a modified version of Figure 28.6 with some of the details of this problem included. CVD Reactor yAniac Y tainethylealli balance =H2 gas