Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help easy to understand 1. In the treatment of wastewater, chlorine (Cl2) gas is mixed with air and bubbled into water. The chlorine dissolves into
help easy to understand 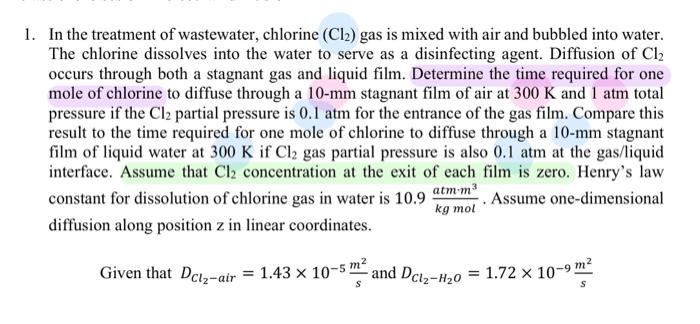
1. In the treatment of wastewater, chlorine (Cl2) gas is mixed with air and bubbled into water. The chlorine dissolves into the water to serve as a disinfecting agent. Diffusion of Cl2 occurs through both a stagnant gas and liquid film. Determine the time required for one mole of chlorine to diffuse through a 10mm stagnant film of air at 300K and 1atm total pressure if the Cl2 partial pressure is 0.1atm for the entrance of the gas film. Compare this result to the time required for one mole of chlorine to diffuse through a 10mm stagnant film of liquid water at 300KifCl2 gas partial pressure is also 0.1atm at the gas/liquid interface. Assume that Cl2 concentration at the exit of each film is zero. Henry's law constant for dissolution of chlorine gas in water is 10.9kgmolatmm3. Assume one-dimensional diffusion along position z in linear coordinates. Given that DC2air=1.43105sm2 and DCl2H2O=1.72109sm2 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


