Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help me Equations 1. Write the equations for the dissociation of each salt in solution 2. For each precipitate formed in the experiment, write an
help me 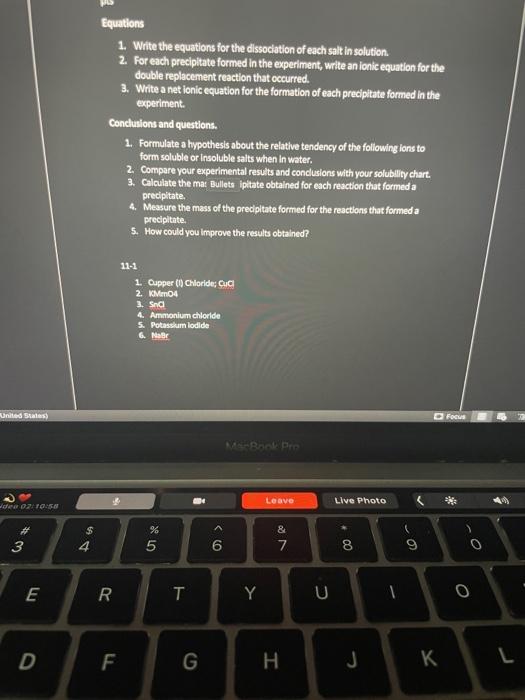
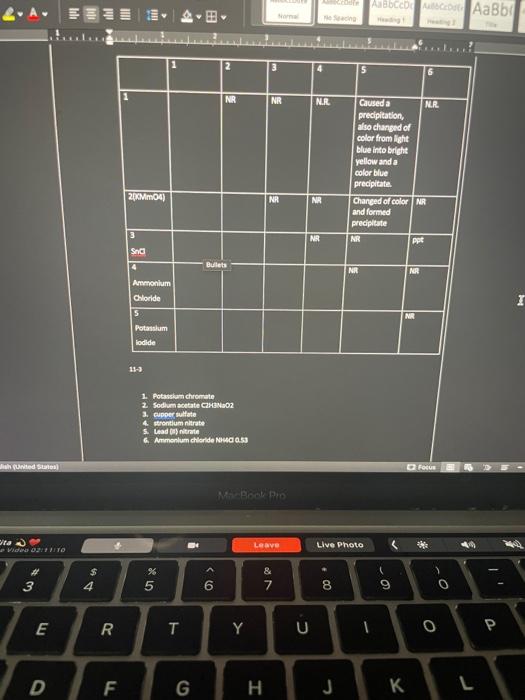
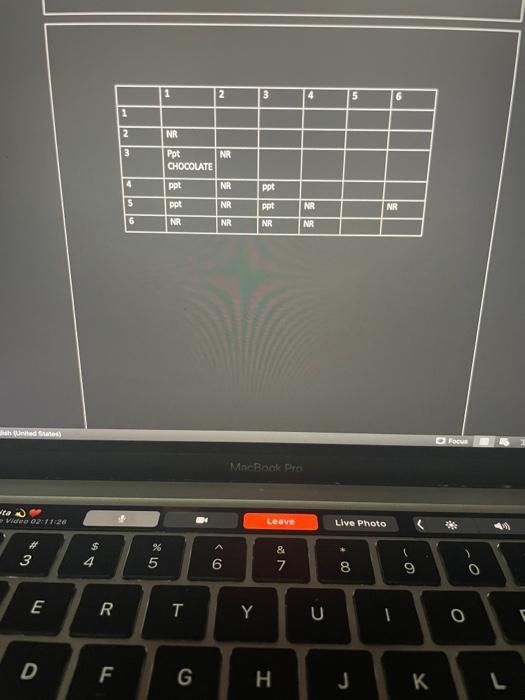
Equations 1. Write the equations for the dissociation of each salt in solution 2. For each precipitate formed in the experiment, write an ionic equation for the double replacement reaction that occurred. 3. Write a net ionic equation for the formation of each precipitate formed in the experiment. Conclusions and questions. 1. Formulate a hypothesis about the relative tendency of the following ions to form soluble or Insoluble salts when in water. 2. Compare your experimental results and conclusions with your solubiley chart. 3. Calculate the mat Bullets ipitate obtained for each reaction that formed a precipitate. 4. Measure the mass of the precipitate formed for the reactions that formed a precipitate 5. How could you improve the results obtained? 11-1 1. Cupper (1) Chloride, CIC 2. KMM04 3. Snd 4. Ammonium chloride 5. Potassium lodide United States D Focus SARA Pre ) Leave Live Photo 02:50 3 00. & 7 - - 6 8 9 0 o E R QC T Y U 0 0 D F G I J 1 2 3 4 5 1 2 NR 3 NR CHOCOLATE 4 ppt NR ppt 5 ppt ppt NR NR NR NR 6 NR NR NR hades Focus MacBook Pro ta Mid 02:1128 Leave Live Photo 2 * 3 $ 4 % 5 6 & 7 8 9 0 E R T Y U 0 D F G H J L Equations 1. Write the equations for the dissociation of each salt in solution 2. For each precipitate formed in the experiment, write an ionic equation for the double replacement reaction that occurred. 3. Write a net ionic equation for the formation of each precipitate formed in the experiment. Conclusions and questions. 1. Formulate a hypothesis about the relative tendency of the following ions to form soluble or Insoluble salts when in water. 2. Compare your experimental results and conclusions with your solubiley chart. 3. Calculate the mat Bullets ipitate obtained for each reaction that formed a precipitate. 4. Measure the mass of the precipitate formed for the reactions that formed a precipitate 5. How could you improve the results obtained? 11-1 1. Cupper (1) Chloride, CIC 2. KMM04 3. Snd 4. Ammonium chloride 5. Potassium lodide United States D Focus SARA Pre ) Leave Live Photo 02:50 3 00. & 7 - - 6 8 9 0 o E R QC T Y U 0 0 D F G I J 1 2 3 4 5 1 2 NR 3 NR CHOCOLATE 4 ppt NR ppt 5 ppt ppt NR NR NR NR 6 NR NR NR hades Focus MacBook Pro ta Mid 02:1128 Leave Live Photo 2 * 3 $ 4 % 5 6 & 7 8 9 0 E R T Y U 0 D F G H J L 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


