Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help me solve this question please Calculate Keq for the reaction in this experiment based on the following equilibrium concentrations. Report your answer to the
help me solve this question please 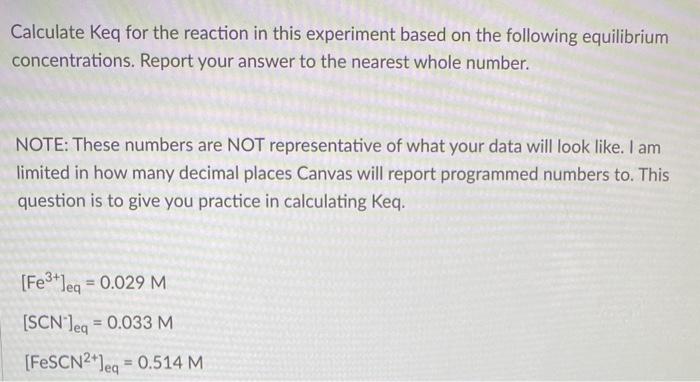
Calculate Keq for the reaction in this experiment based on the following equilibrium concentrations. Report your answer to the nearest whole number. NOTE: These numbers are NOT representative of what your data will look like. I am limited in how many decimal places Canvas will report programmed numbers to. This question is to give you practice in calculating Keq. [Fe3+]eq=0.029M[SCN]eq=0.033M[FeSCN2+]eq=0.514M 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


