Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help me to solve this pls e the equation below to calculate the following: 3Ca(NO3)2(aq)+2Na3PO4(aq)Ca3(PO4)2(s)+6NaNO3(aq) (a) the moles Ca3(PO4)2 produced from 2.7molNa3PO4 molCaCa3(PO4)2 (b) the
help me to solve this pls 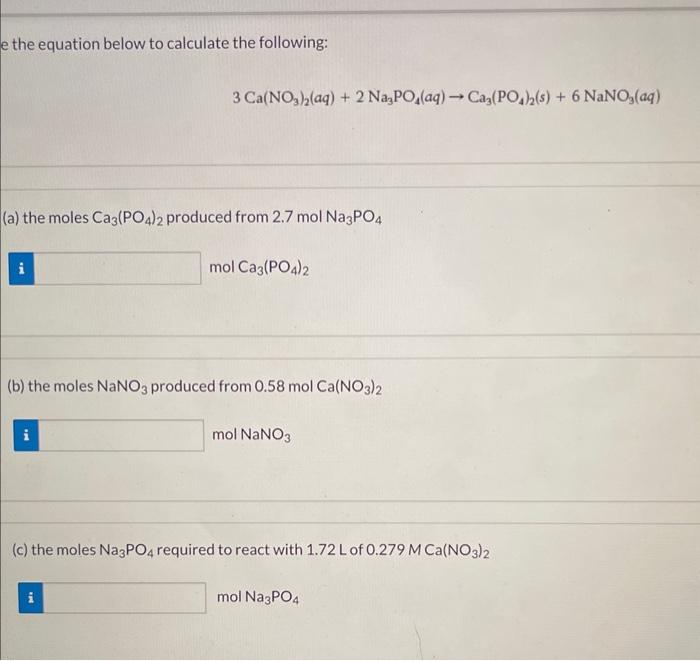

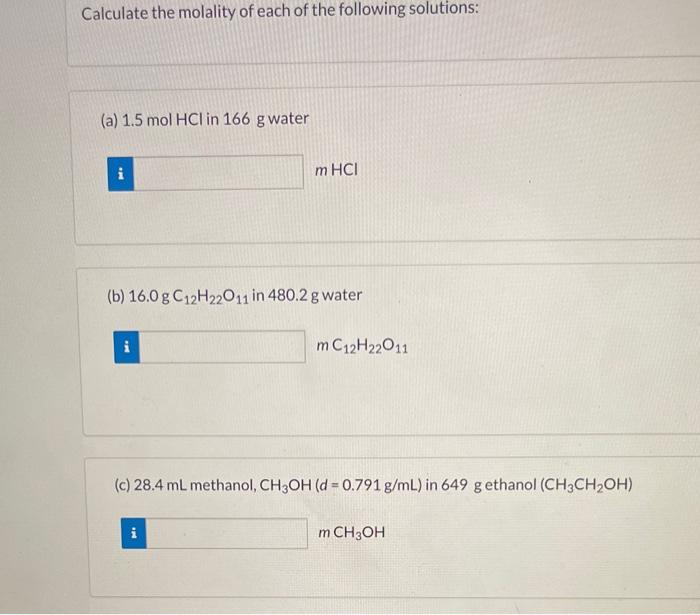
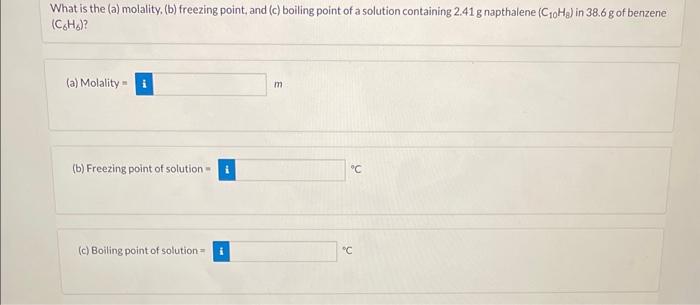
e the equation below to calculate the following: 3Ca(NO3)2(aq)+2Na3PO4(aq)Ca3(PO4)2(s)+6NaNO3(aq) (a) the moles Ca3(PO4)2 produced from 2.7molNa3PO4 molCaCa3(PO4)2 (b) the moles NaNO3 produced from 0.58molCa(NO3)2 molNaNO3 (c) the moles Na3PO4 required to react with 1.72L of 0.279MCa(NO3)2 molNaNO4 (d) the grams of Ca3(PO4)2 that can be obtained from 121mL of 0.674MCa(NO3)2 gCa(PO4)2 (e) the volume of 0.16MNa3PO4 needed to react with 24mLof0.36MCa(NO3)2 mLNa3PO4 (f) the molarity (M) of the Ca(NO3)2 solution when 58.5mL react with 35.5mL of 4.9MNa3PO4 MCa(NO3)2 Calculate the molality of each of the following solutions: (a) 1.5molHCl in 166 g water mHCl (b) 16.0gC12H22O11 in 480.2g water mC12H22O11 (c) 28.4mL methanol, CH3OH(d=0.791g/mL) in 649g ethanol (CH3CH2OH) mCH3OH What is the (a) molality. (b) freezing point, and (c) boiling point of a solution containing 2.41g napthalene (C10H8) in 38.6g of benzene (C6H6) ? (a) Molality = m (b) Freezing point of solution = 9C (c) Boiling point of solution = 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


