Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Help plz. 9.5- Electronegativity 1. Determine the type of bond (ionic, polar covalent, or non-polar covalent) that will form between atoms of the following elements
Help plz.

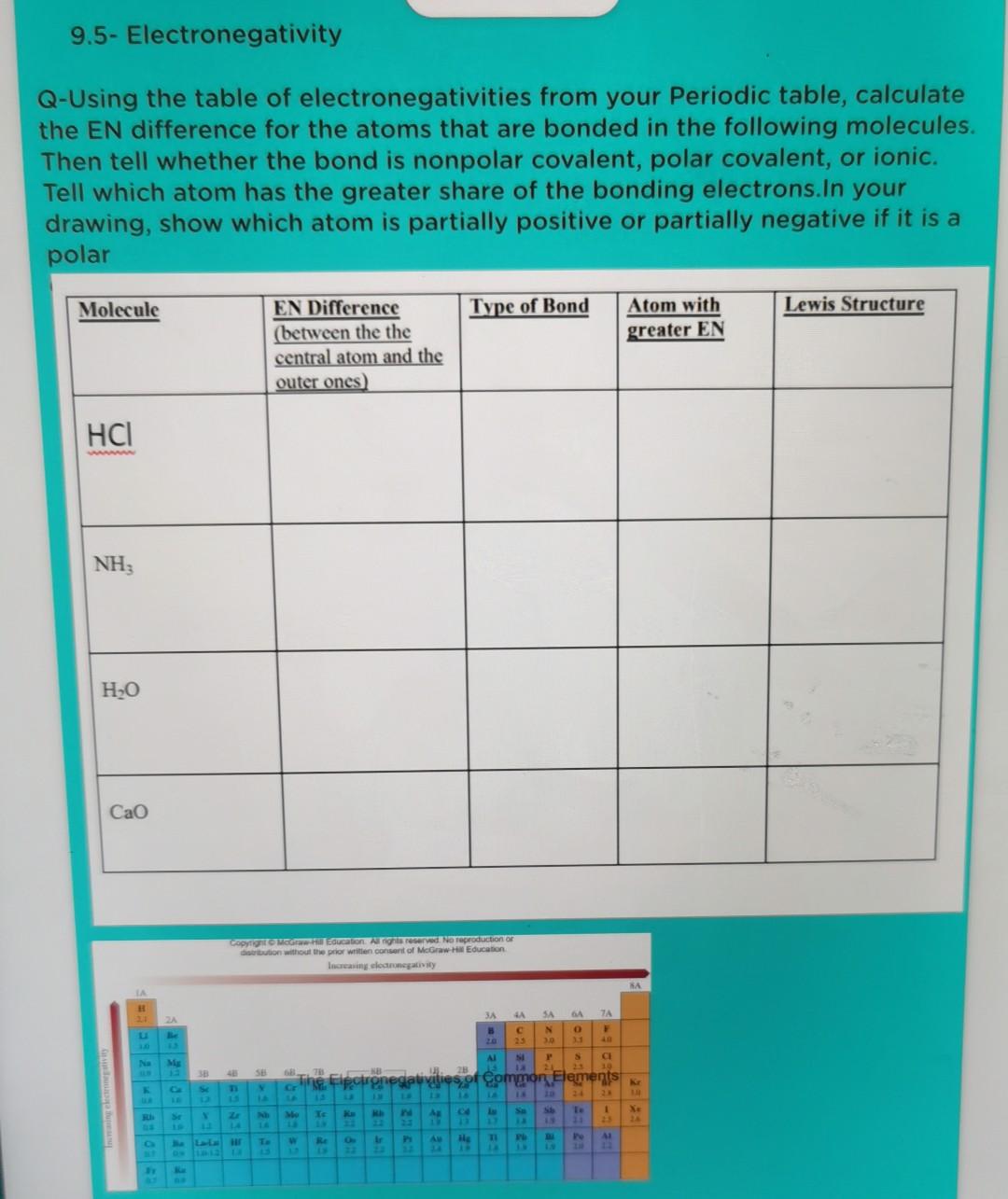
9.5- Electronegativity 1. Determine the type of bond (ionic, polar covalent, or non-polar covalent) that will form between atoms of the following elements and show the polarity of the bond if it is polar covalent. a. Ca and Ci lonic b. Cand s c. Mg and F d. N and O e. H and o f. S and o 9.5- Electronegativity Q-Using the table of electronegativities from your Periodic table, calculate the EN difference for the atoms that are bonded in the following molecules. Then tell whether the bond is nonpolar covalent, polar covalent, or ionic. Tell which atom has the greater share of the bonding electrons.In your drawing, show which atom is partially positive or partially negative if it is a polar Molecule Type of Bond Lewis Structure Atom with greater EN EN Difference (between the the central atom and the outer ones) HCI NH; HO Cao Sopron Meducation. Ang reserved. No reproduction or dation without the prior written consent of McGraw Education Increasing electronegativity SA H 3A TA 2 LA 23 B SA N 3.0 OB 40 AI SI 5 Ne CI 19 28 2 FIRE The Flpatronegativities of Common Elements Te AR SI Se 1 X ELE AN TI PL 41
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


