Answered step by step
Verified Expert Solution
Question
1 Approved Answer
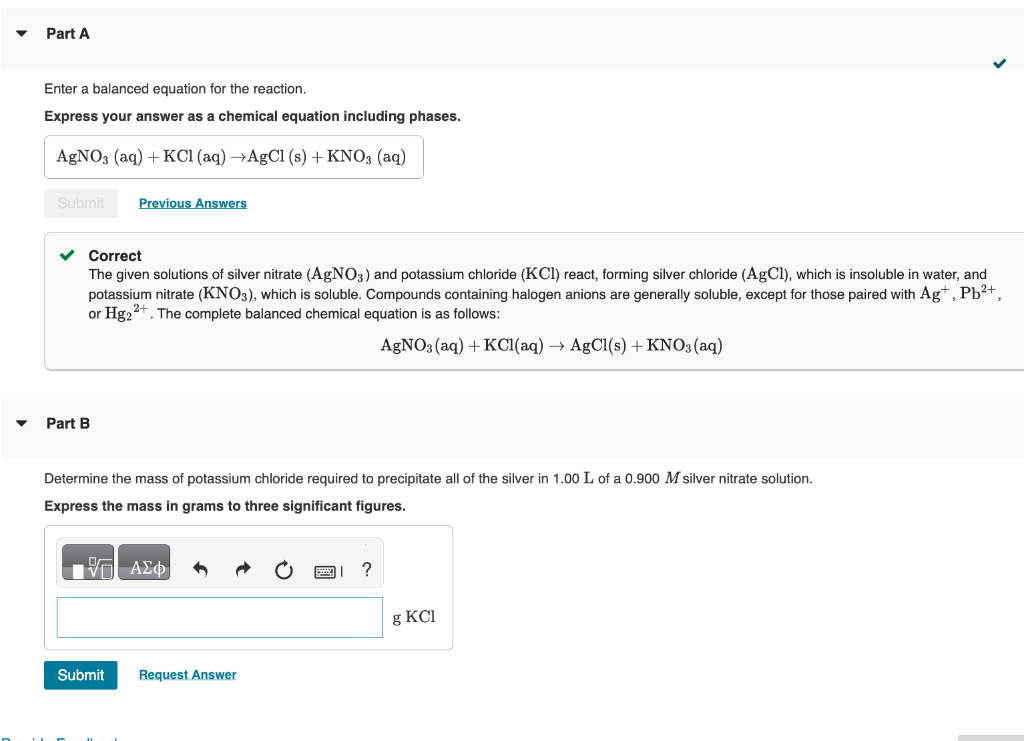
help solve part b Enter a balanced equation for the reaction. Express your answer as a chemical equation including phases. Correct The given solutions of
help solve part b
Enter a balanced equation for the reaction. Express your answer as a chemical equation including phases. Correct The given solutions of silver nitrate (AgNO3) and potassium chloride (KCl) react, forming silver chloride ( AgCl), which is insoluble in water, and potassium nitrate (KNO3), which is soluble. Compounds containing halogen anions are generally soluble, except for those paired with Ag+,Pb2+, or Hg22+. The complete balanced chemical equation is as follows: AgNO3(aq)+KCl(aq)AgCl(s)+KNO3(aq) Part B Determine the mass of potassium chloride required to precipitate all of the silver in 1.00L of a 0.900M silver nitrate solution. Express the mass in grams to three significant figuresStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


