Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help with 3 and 4 Determine the freezing point of an aqueous solution prepared by adding 185.4mg of saccharin (C7H5O3NS) to 1.35mL of pure water
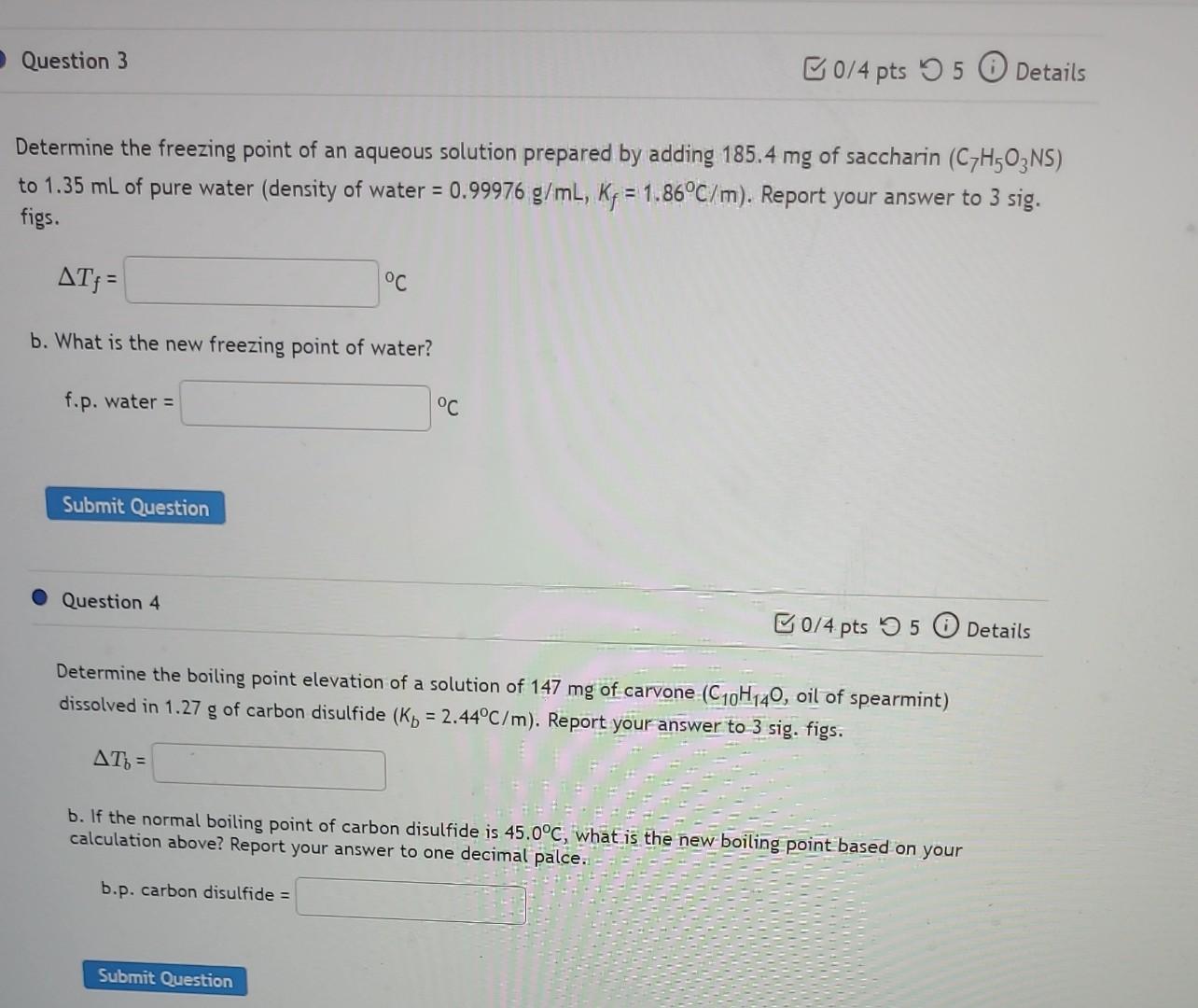
help with 3 and 4
Determine the freezing point of an aqueous solution prepared by adding 185.4mg of saccharin (C7H5O3NS) to 1.35mL of pure water (density of water =0.99976g/mL,Kf=1.86C/m ). Report your answer to 3 sig. figs. Tf= b. What is the new freezing point of water? f.p.water= Question 4 0/4.pts 55 (i) Details Determine the boiling point elevation of a solution of 147mg of carvone (C10H14O, oil of spearmint) dissolved in 1.27g of carbon disulfide (Kb=2.44C/m). Report your answer to 3 sig. figs. Tb= b. If the normal boiling point of carbon disulfide is 45.0C, what is the new boiling point based on your calculation above? Report your answer to one decimal palce. b.p. carbon disulfide =Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


