Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Help with last 3? For ATM I tried 0.00062 x 0.0821 x 297.15 divided by 0.01500 and got 1.008 but this incorrect? Thanks Lab Data
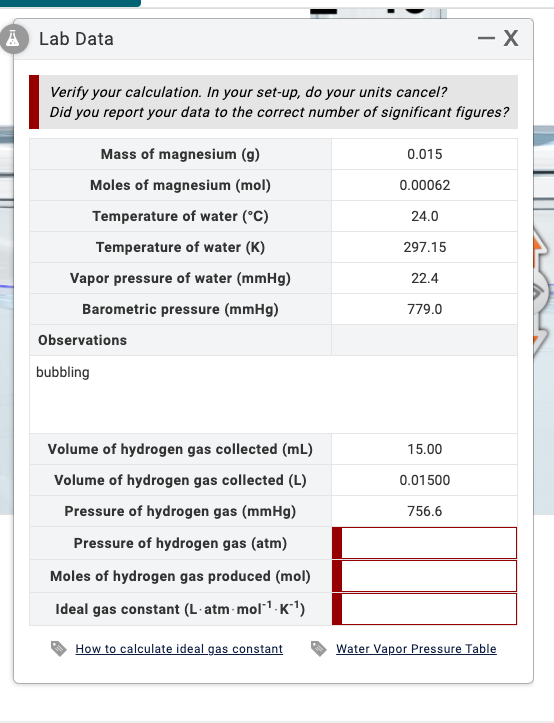
Help with last 3?
For ATM I tried 0.00062 x 0.0821 x 297.15 divided by 0.01500 and got 1.008 but this incorrect?
Thanks
Lab Data - X Verify your calculation. In your set-up, do your units cancel? Did you report your data to the correct number of significant figures? 0.015 Mass of magnesium (g) Moles of magnesium (mol) Temperature of water (C) 0.00062 24.0 Temperature of water (K) 297.15 22.4 Vapor pressure of water (mmHg) Barometric pressure (mmHg) Observations 779.0 bubbling 15.00 0.01500 756.6 Volume of hydrogen gas collected (mL) Volume of hydrogen gas collected (L) Pressure of hydrogen gas (mmHg) Pressure of hydrogen gas (atm) Moles of hydrogen gas produced (mol) Ideal gas constant (L-atm-mol-K'') How to calculate ideal gas constant Water Vapor Pressure TableStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


