Answered step by step
Verified Expert Solution
Question
1 Approved Answer
help with the following pls Au3+(aq)+3eAu(s),Er=+1.50VSn2+(aq)+2eSn(s),Er=0.14VCo2+(aq)+2eCo(s),Er=0.28VCa2+2aq)+2eCa(s),Er=2.76V From the reactions above, a spontaneous reaction will occur if gold ion reacts with what? calcium metal cobalt metal
help with the following pls 
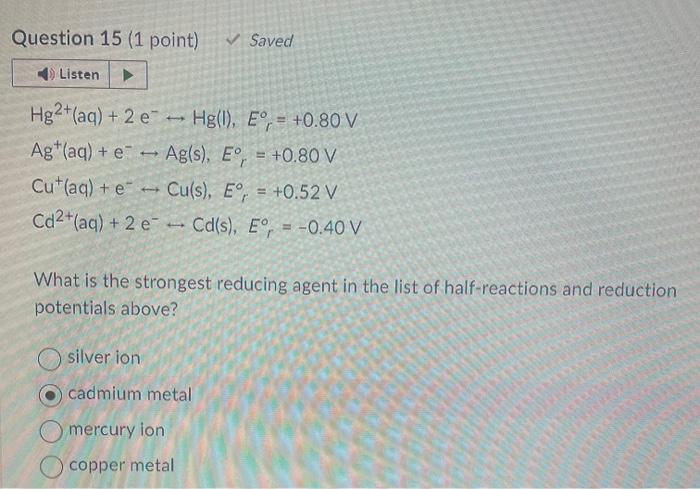
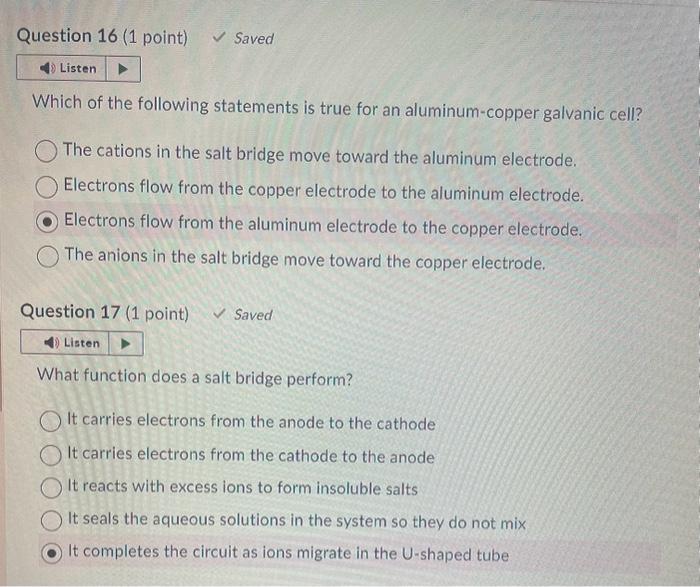
Au3+(aq)+3eAu(s),Er=+1.50VSn2+(aq)+2eSn(s),Er=0.14VCo2+(aq)+2eCo(s),Er=0.28VCa2+2aq)+2eCa(s),Er=2.76V From the reactions above, a spontaneous reaction will occur if gold ion reacts with what? calcium metal cobalt metal all of the above tin metal Hg2+(aq)+2eHg(l),Er0=+0.80VAg+(aq)+eAg(s),Er=+0.80VCu+(aq)+eCu(s),Er=+0.52VCd2+(aq)+2eCd(s),Er=0.40V What is the strongest reducing agent in the list of half-reactions and reduction potentials above? silver ion cadmium metal mercury ion copper metal Which of the following statements is true for an aluminum-copper galvanic cell? The cations in the salt bridge move toward the aluminum electrode. Electrons flow from the copper electrode to the aluminum electrode. Electrons flow from the aluminum electrode to the copper electrode. The anions in the salt bridge move toward the copper electrode. Question 17 (1 point) Saved What function does a salt bridge perform? It carries electrons from the anode to the cathode It carries electrons from the cathode to the anode It reacts with excess ions to form insoluble salts It seals the aqueous solutions in the system so they do not mix It completes the circuit as ions migrate in the U-shaped tube 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


