Question
Here we assume that the hydrogenation of C,H, to CH, goes through the mechanism: (1) CH * +2 (2) CH + H. * (3)
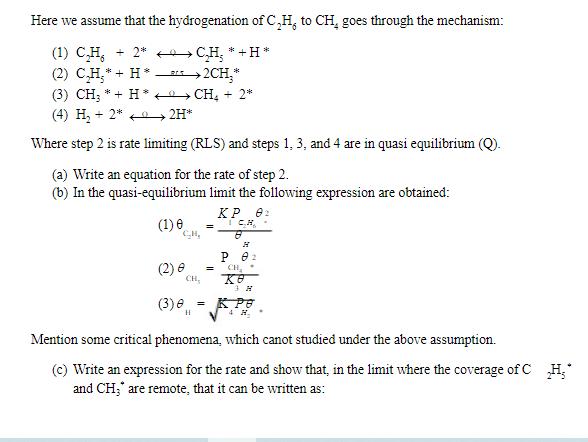
Here we assume that the hydrogenation of C,H, to CH, goes through the mechanism: (1) CH * +2 (2) CH + H. * (3) CH3 + H (4) H+2* CH *+H* >2CH3* CH4 +2* >2H* Where step 2 is rate limiting (RLS) and steps 1, 3, and 4 are in quasi equilibrium (Q). (a) Write an equation for the rate of step 2. (b) In the quasi-equilibrium limit the following expression are obtained: (1) 0 (2) 8 CH, CH, = KP 02 8 H 02 = CH H (3)e = K Pe H Mention some critical phenomena, which canot studied under the above assumption. (c) Write an expression for the rate and show that, in the limit where the coverage of C H and CH;*" are remote, that it can be written as:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Business Statistics In Practice
Authors: Bruce Bowerman, Richard O'Connell
6th Edition
0073401838, 978-0073401836
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



