Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Homework # 1 Use the Davies equation as shown below for calculating mean ionic activity coefficients needed in working the following problems. Davies equation: ,
Homework #
Use the Davies equation as shown below for calculating mean ionic activity coefficients needed in working the following problems.
Davies equation:
where and represents molality and is the charge.
What is the ionic strength of a solution prepared by dissolving of and in of water?
~~
Estimate the mean ionic activity coefficient for in the solution from problem
~~
What is the molality of in the solution from problem at Do not neglect ionic activity coefficients.
Formic acid, is a weak acid with What is the molality of ions in a solution of formic acid in water? Do not neglect ionic activity coefficients.
Just need and
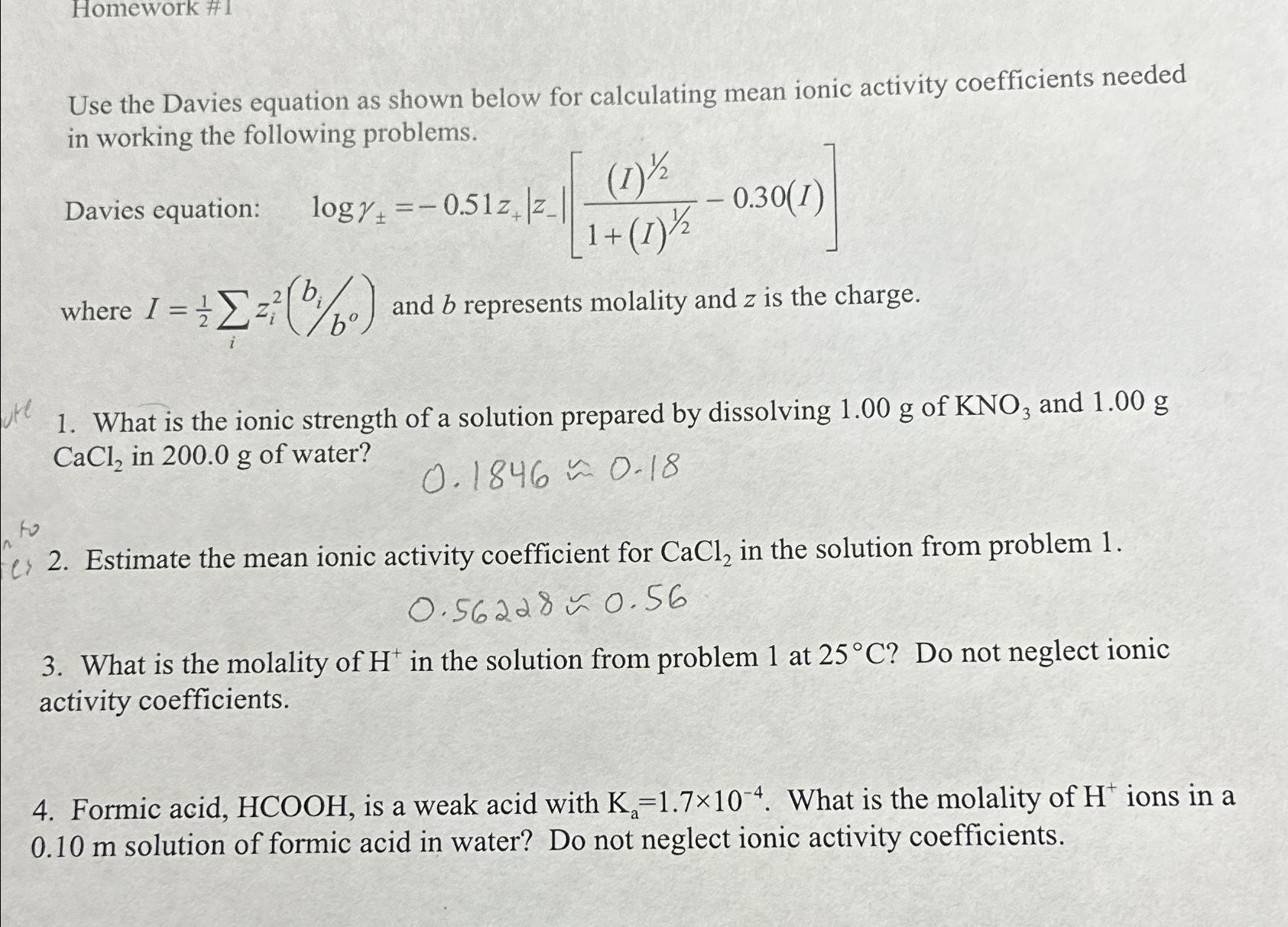
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


