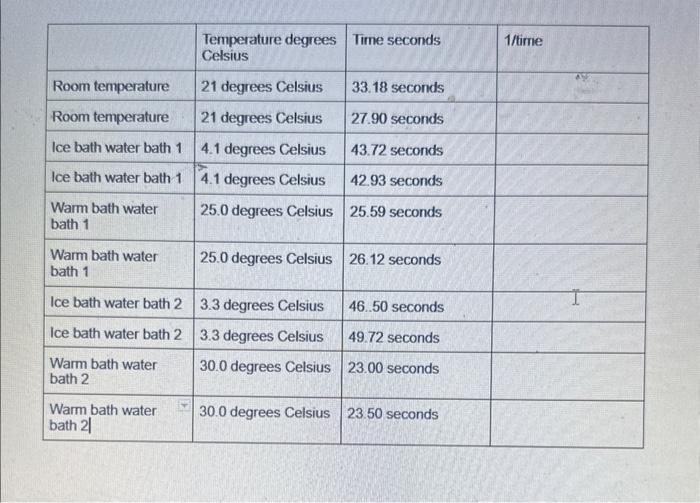
how do you calculate the the 1/time for each trial?
Report This is the first part of three experiments each of which studies the same chemical system. All three parts will be turned in together. You will receive a summary page of things to keep in mind when preparing the three reports. Your report for this part (part 1) of the experiment will consist of the following: 1. Organized data table: Construct your own data table after recording your raw data today. The first column should be the independent variable. In this case that is temperature in C. Arrange your data systematically - in order of increasing or decreasing temperature. The table should have three columns, temperature, time, and 1/ time with appropriate units noted. A data table should have an identifying title. 2. A graph: Plot temperature (C) as the independent variable ( x-axis) and the reciprocal of the time as the dependent variable. The resulting plot should appear in the form of a smooth curve. Since time and rate are inversely proportional and since the actual rate of this reaction is not being measured, you may use 1/t instead of the actual rate. 3. An interpretation of the graph: A one or two sentence summary of what you learn about reaction rates from your graph. Do not simply restate what you have plotted. The reaction may be represented by the following equations: HIO3+3H2SO3HIO3+5HI2H2O+2I2+2H2SO3HI+3H2SO43H2O+3I22H2SO4+4HI Although the system is actually rather complex, the rate constant may be calculated as though the reaction were of the simple type: A+BC+D in which A is HIO3 and B is H2SO3. \begin{tabular}{|l|l|l|l|} \hline & TemperaturedegreesCelsius & Time seconds & 1 ttime \\ \hline Room temperature & 21 degrees Celsius & 33.18 seconds & \\ \hline Room temperature & 21 degrees Celsius & 27.90 seconds & \\ \hline Ice bath water bath 1 & 4.1 degrees Celsius & 43.72 seconds & \\ \hline Ice bath water bath 1 & 4.1 degrees Celsius & 42.93 seconds & \\ \hline Warmbathwaterbath1 & 25.0 degrees Celsius & 25.59 seconds & \\ \hline Warmbathwaterbath1 & 25.0 degrees Celsius & 26.12 seconds & \\ \hline Ice bath water bath 2 & 3.3 degrees Celsius & 46.50 seconds & \\ \hline Ice bath water bath 2 & 3.3 degrees Celsius & 49.72 seconds & \\ \hline Warmbathwaterbath2 & 30.0 degrees Celsius & 23.00 seconds & \\ \hline Warmbathwaterbath2l & 30.0 degrees Celsius & 23.50 seconds & \\ \hline \end{tabular} Procedure You will measure the speed of the reaction at five different temperatures, and will test each temperature twice (= 10 trials in total). Set up 2 water baths using two nested Styrofoam cups for each bath. Use a mix of tap water and a small amount of ice. The colder bath should be around 78C, and the other should fall between that and room temperature (e.g., around 1415C ) The other two temperatures (warmer than room temperature). will be achieved using the incubators on the lab benches. Record the temperature on the display for each. From the bottles, measure 5.00mL of each acid solution into separate clean, dry test tubes and add 23 drops of starch solution to each test tube. Stopper the test tube containing the sulfurous acid solution. Prepare samples for all 10 trials ( =10 with HIO3,10 with H2SO3 ). 4. To maintain a given temperature, immerse the test tubes in one of the water baths and allow them to stand until they are the same temperature as the bath ( 5 to 10 minutes). 5. While the other samples are equilibrating in the water baths, run the two room temperature trials: quickly pour the sulfurous acid solution into the iodic acid solution and back into the empty tube. Pour them back and forth 34 times taking care not to spill any of the acids. Re-stopper the test tube, shake it and return it to the water bath (when applicable). You must start timing at the instant the two solutions meet, and stop timing as soon as the first color appears. If the color appears in one part of the solution before it does in other parts, you did not mix the solutions thoroughly and you should repeat the trial. Use the stopwatch (not your phone) to time the reaction. Record all digits from the stopwatch. Report This is the first part of three experiments each of which studies the same chemical system. All three parts will be turned in together. You will receive a summary page of things to keep in mind when preparing the three reports. Your report for this part (part 1) of the experiment will consist of the following: 1. Organized data table: Construct your own data table after recording your raw data today. The first column should be the independent variable. In this case that is temperature in C. Arrange your data systematically - in order of increasing or decreasing temperature. The table should have three columns, temperature, time, and 1/ time with appropriate units noted. A data table should have an identifying title. 2. A graph: Plot temperature (C) as the independent variable ( x-axis) and the reciprocal of the time as the dependent variable. The resulting plot should appear in the form of a smooth curve. Since time and rate are inversely proportional and since the actual rate of this reaction is not being measured, you may use 1/t instead of the actual rate. 3. An interpretation of the graph: A one or two sentence summary of what you learn about reaction rates from your graph. Do not simply restate what you have plotted. The reaction may be represented by the following equations: HIO3+3H2SO3HIO3+5HI2H2O+2I2+2H2SO3HI+3H2SO43H2O+3I22H2SO4+4HI Although the system is actually rather complex, the rate constant may be calculated as though the reaction were of the simple type: A+BC+D in which A is HIO3 and B is H2SO3. \begin{tabular}{|l|l|l|l|} \hline & TemperaturedegreesCelsius & Time seconds & 1 ttime \\ \hline Room temperature & 21 degrees Celsius & 33.18 seconds & \\ \hline Room temperature & 21 degrees Celsius & 27.90 seconds & \\ \hline Ice bath water bath 1 & 4.1 degrees Celsius & 43.72 seconds & \\ \hline Ice bath water bath 1 & 4.1 degrees Celsius & 42.93 seconds & \\ \hline Warmbathwaterbath1 & 25.0 degrees Celsius & 25.59 seconds & \\ \hline Warmbathwaterbath1 & 25.0 degrees Celsius & 26.12 seconds & \\ \hline Ice bath water bath 2 & 3.3 degrees Celsius & 46.50 seconds & \\ \hline Ice bath water bath 2 & 3.3 degrees Celsius & 49.72 seconds & \\ \hline Warmbathwaterbath2 & 30.0 degrees Celsius & 23.00 seconds & \\ \hline Warmbathwaterbath2l & 30.0 degrees Celsius & 23.50 seconds & \\ \hline \end{tabular} Procedure You will measure the speed of the reaction at five different temperatures, and will test each temperature twice (= 10 trials in total). Set up 2 water baths using two nested Styrofoam cups for each bath. Use a mix of tap water and a small amount of ice. The colder bath should be around 78C, and the other should fall between that and room temperature (e.g., around 1415C ) The other two temperatures (warmer than room temperature). will be achieved using the incubators on the lab benches. Record the temperature on the display for each. From the bottles, measure 5.00mL of each acid solution into separate clean, dry test tubes and add 23 drops of starch solution to each test tube. Stopper the test tube containing the sulfurous acid solution. Prepare samples for all 10 trials ( =10 with HIO3,10 with H2SO3 ). 4. To maintain a given temperature, immerse the test tubes in one of the water baths and allow them to stand until they are the same temperature as the bath ( 5 to 10 minutes). 5. While the other samples are equilibrating in the water baths, run the two room temperature trials: quickly pour the sulfurous acid solution into the iodic acid solution and back into the empty tube. Pour them back and forth 34 times taking care not to spill any of the acids. Re-stopper the test tube, shake it and return it to the water bath (when applicable). You must start timing at the instant the two solutions meet, and stop timing as soon as the first color appears. If the color appears in one part of the solution before it does in other parts, you did not mix the solutions thoroughly and you should repeat the trial. Use the stopwatch (not your phone) to time the reaction. Record all digits from the stopwatch