Question
How many atoms of tin are in a 36.5 g sample? Calculate the molar mass of the explosive TNT, C6HCH3 (NO2)3. Calculate the molar
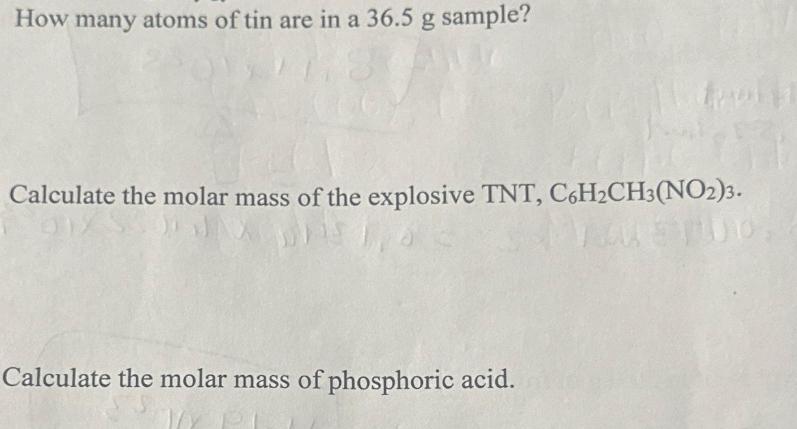
How many atoms of tin are in a 36.5 g sample? Calculate the molar mass of the explosive TNT, C6HCH3 (NO2)3. Calculate the molar mass of phosphoric acid.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The image shows a written question asking to calculate the molar mass of the explosive TNT with the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles The Quest For Insight
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
7th Edition
1464183953, 9781464183959
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



