Question
How many grams of sucrose (C12H22O11) are in 1.55 L of 0.758 M sucrose solution? Express your answer in grams to three significant figures.
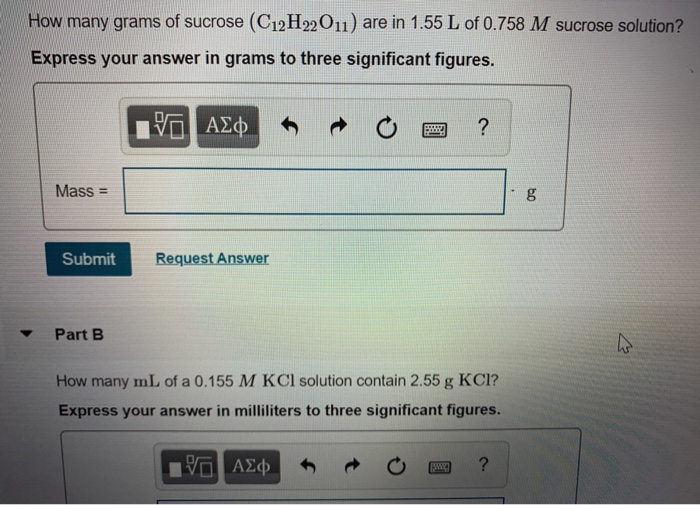
How many grams of sucrose (C12H22O11) are in 1.55 L of 0.758 M sucrose solution? Express your answer in grams to three significant figures. 17 Mass - Submit Request Answer Part B ? How many mL of a 0.155 M KCl solution contain 2.55 g KCI? Express your answer in milliliters to three significant figures. ? to g
Step by Step Solution
3.57 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Answer to part A To answer this question we first need to find two things that has not been given in ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



