Answered step by step
Verified Expert Solution
Question
1 Approved Answer
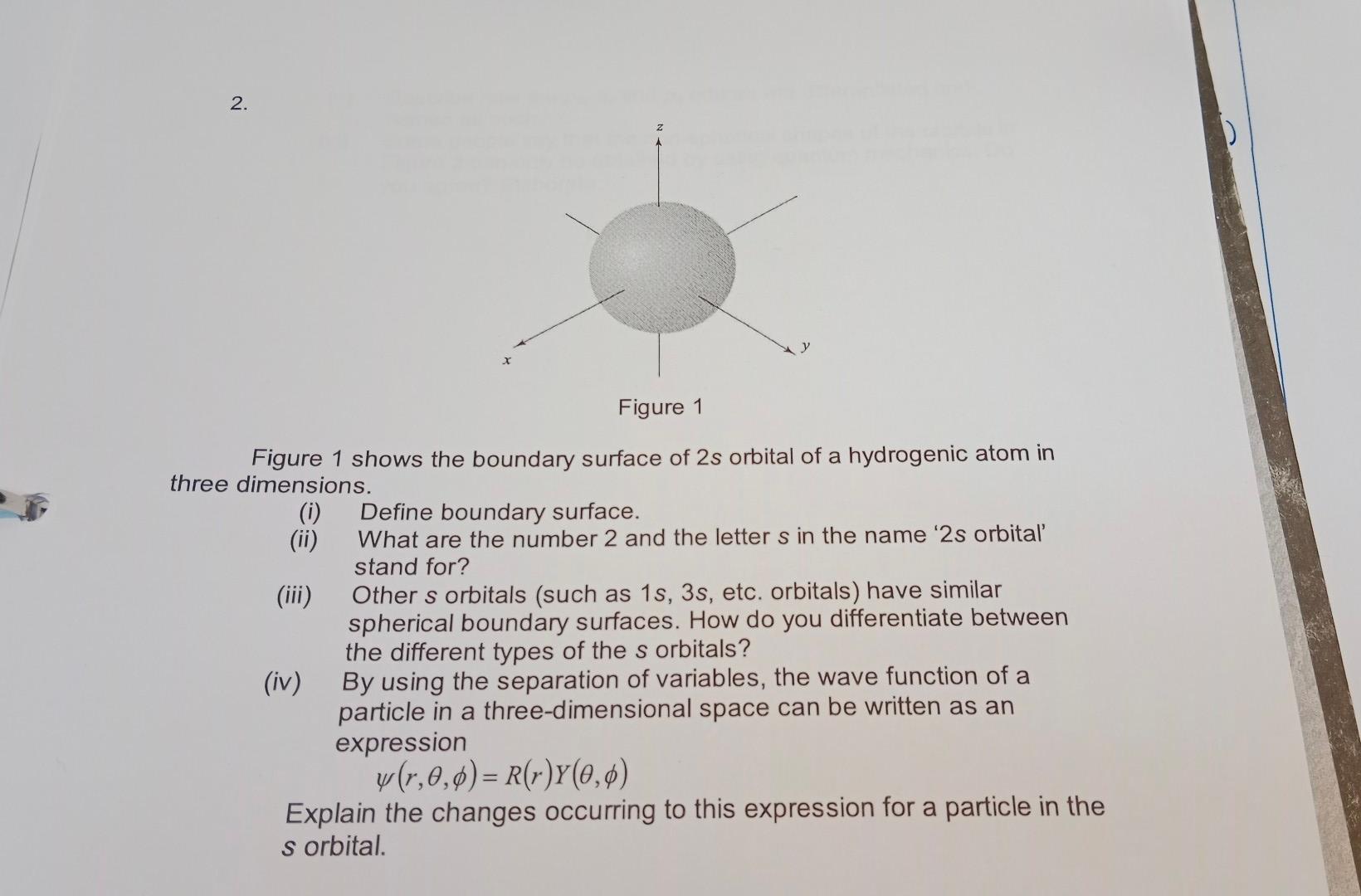
How to solve Q:2 2. y X Figure 1 Figure 1 shows the boundary surface of 2s orbital of a hydrogenic atom in three dimensions.
How to solve Q:2
2. y X Figure 1 Figure 1 shows the boundary surface of 2s orbital of a hydrogenic atom in three dimensions. (0) Define boundary surface. (ii) What are the number 2 and the letter s in the name '2s orbital stand for? (iii) Other s orbitals (such as 1s, 3s, etc. orbitals) have similar spherical boundary surfaces. How do you differentiate between the different types of the s orbitals? (iv) By using the separation of variables, the wave function of a particle in a three-dimensional space can be written as an expression w(r,0,0)= R(r)Y(0,0) Explain the changes occurring to this expression for a particle in the s orbitalStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


