Question
Human serum albumin (HSA) has a molecular weight of 66,000 g/mol. One compartment contains a solution with HSA and the other compartment in the
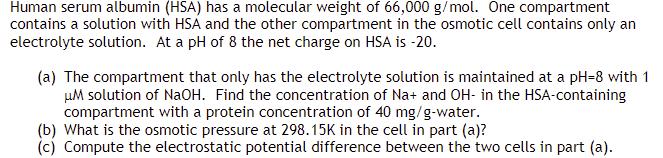
Human serum albumin (HSA) has a molecular weight of 66,000 g/mol. One compartment contains a solution with HSA and the other compartment in the osmotic cell contains only an electrolyte solution. At a pH of 8 the net charge on HSA is -20. (a) The compartment that only has the electrolyte solution is maintained at a pH-8 with 1 M solution of NaOH. Find the concentration of Na+ and OH- in the HSA-containing compartment with a protein concentration of 40 mg/g-water. (b) What is the osmotic pressure at 298.15K in the cell in part (a)? (c) Compute the electrostatic potential difference between the two cells in part (a).
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer Lets solve each part step by step a Concentration of Na and OH in the HSAcontaining compartme...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry Principles And Modern Applications
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
11th Edition
0132931281, 978-0132931281
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



