Question
(A) A liquid mixture with x1 = 0.6 at 8 bar and 400 K is isothermally decompressed until the first little bubble of vapor forms.
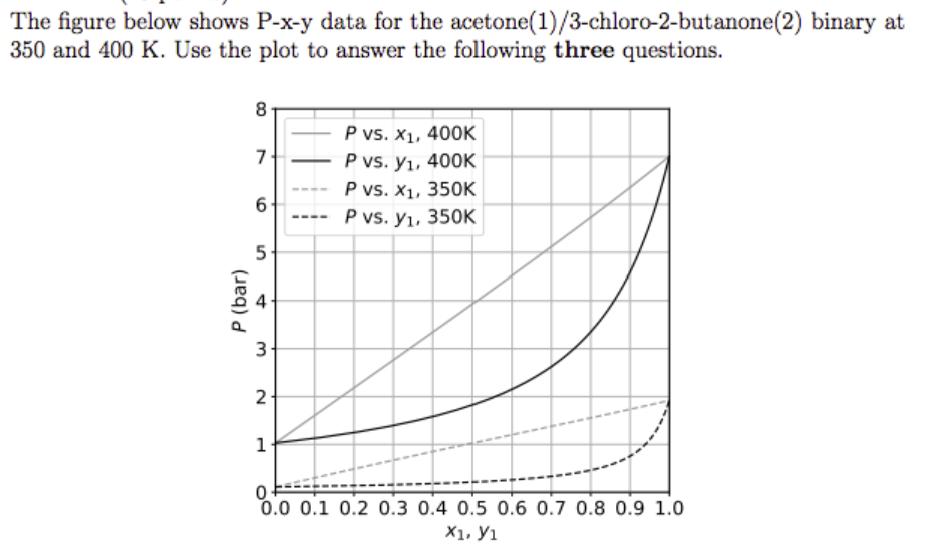
(A) A liquid mixture with x1 = 0.6 at 8 bar and 400 K is isothermally decompressed until the first little bubble of vapor forms. What is the pressure at this point?
(B) Once this first bubble is formed, the temperature is held fixed and the pressure is adjusted to 3.0 bar. What are the compositions of the two phases that form?
(C) The vapor phase from part (B) is fed to a flash unit operating at 350 K and 1.0 bar. Does this mixture flash? If so, estimate the composition of the two product phases and the fraction of the inlet liquid that vaporizes.
While solving this question please sketch the provided P-x-y data and show work.
The figure below shows P-x-y data for the acetone (1)/3-chloro-2-butanone(2) binary at 350 and 400 K. Use the plot to answer the following three questions. P (bar) 8 7- 6 5- t 3- 2 1 - P vs. X, 400K P vs. y, 400K P vs. x, 350K P vs. y, 350K 0 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 X1. 1
Step by Step Solution
3.52 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Solution for the ab...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


