Question
Consider a high-pressure vessel at 25 bar. Inside the vessel, there is a thermostat containing 1 L of liquid water at 298 K. This
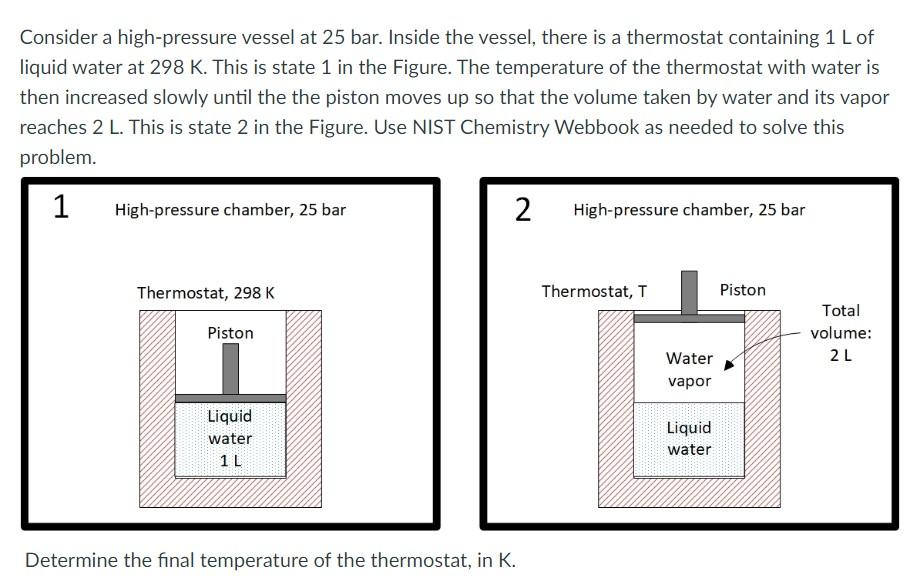
Consider a high-pressure vessel at 25 bar. Inside the vessel, there is a thermostat containing 1 L of liquid water at 298 K. This is state 1 in the Figure. The temperature of the thermostat with water is then increased slowly until the the piston moves up so that the volume taken by water and its vapor reaches 2 L. This is state 2 in the Figure. Use NIST Chemistry Webbook as needed to solve this problem. 1 High-pressure chamber, 25 bar Thermostat, 298 K Piston Liquid water 1 L 2 Determine the final temperature of the thermostat, in K. High-pressure chamber, 25 bar Thermostat, T Water vapor Liquid water Piston Total volume: 2 L
Step by Step Solution
3.39 Rating (143 Votes )
There are 3 Steps involved in it
Step: 1
We can start by identifying the system as a closed system meaning that no matter can enter or leave ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau
3rd Edition
978-0471687573, 9788126515820, 978-0-471-4152, 0471720631, 047168757X, 8126515821, 978-0471720638
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



