Hydrazine (NH4) is a highly toxic and unstable monopropellant that has been used as a rocket fuel in various applications. With help from a
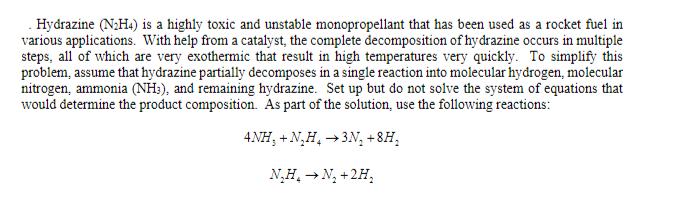
Hydrazine (NH4) is a highly toxic and unstable monopropellant that has been used as a rocket fuel in various applications. With help from a catalyst, the complete decomposition of hydrazine occurs in multiple steps, all of which are very exothermic that result in high temperatures very quickly. To simplify this problem, assume that hydrazine partially decomposes in a single reaction into molecular hydrogen, molecular nitrogen, ammonia (NH3), and remaining hydrazine. Set up but do not solve the system of equations that would determine the product composition. As part of the solution, use the following reactions: ANH,+N_H,3N+8H_ NH N +2H
Step by Step Solution
3.26 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
To set up the system of equations for the partial decomposition of hydrazine N2H4 into molecular hyd...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


