Answered step by step
Verified Expert Solution
Question
1 Approved Answer
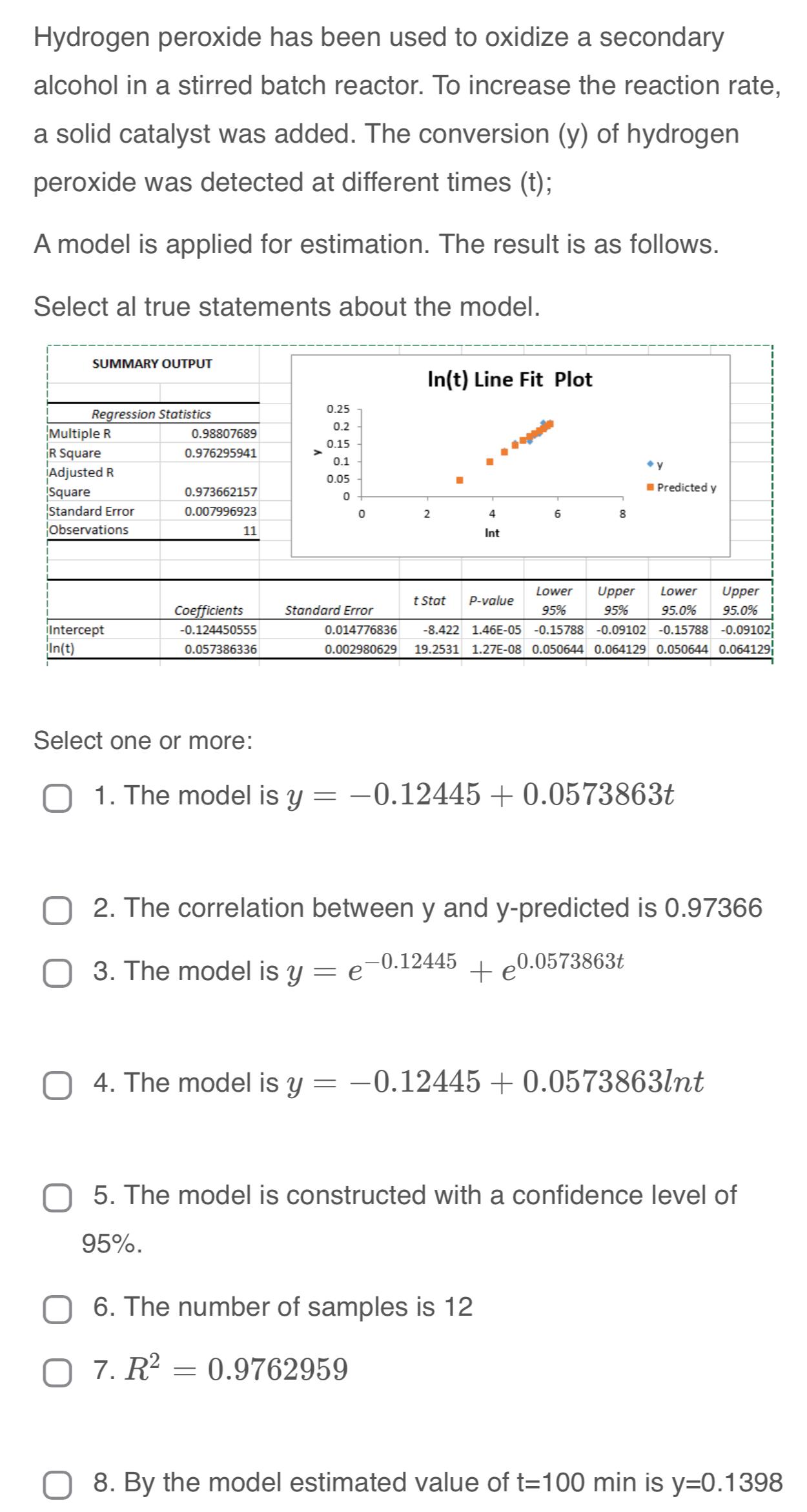
Hydrogen peroxide has been used to oxidize a secondary alcohol in a stirred batch reactor. To increase the reaction rate, a solid catalyst was added.
Hydrogen peroxide has been used to oxidize a secondary alcohol in a stirred batch reactor. To increase the reaction rate, a solid catalyst was added. The conversion of hydrogen peroxide was detected at different times ;
A model is applied for estimation. The result is as follows.
Select al true statements about the model.
Select one or more:
The model is
The correlation between y and ypredicted is
The model is
The model is
The model is constructed with a confidence level of
The number of samples is
By the model estimated value of min is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


