Question
I am asked to plot the acetone composition in the vapor vs. the liquid composition. How can I do that? and how to I find
I am asked to plot the acetone composition in the vapor vs. the liquid composition. How can I do that? and how to I find the azeotrope using the graph?
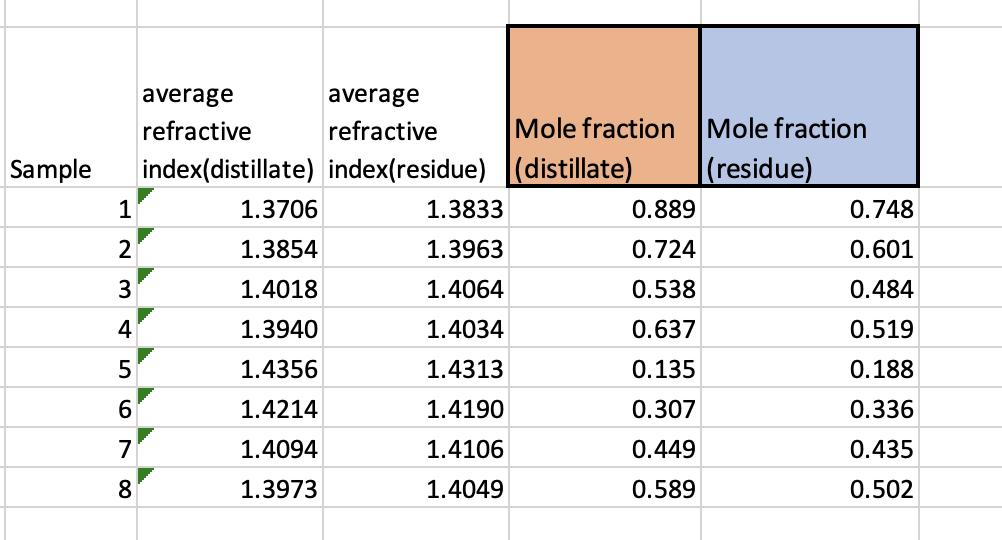
average refractive average refractive Mole fraction Mole fraction Sample index(distillate) index(residue) (distillate) (residue) 1 1.3706 1.3833 0.889 0.748 23 2 1.3854 1.3963 0.724 0.601 3 1.4018 1.4064 0.538 0.484 4 1.3940 1.4034 0.637 0.519 567 1.4356 1.4313 0.135 0.188 1.4214 1.4190 0.307 0.336 1.4094 1.4106 0.449 0.435 8. 1.3973 1.4049 0.589 0.502
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Separation process principles
Authors: J. D. Seader
2nd Edition
471464805, 978-0471464808
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



