Question: A tower, packed with Montz B1-200 metal structured packing, is to be designed to absorb SO2 from air by scrubbing with water. The entering gas,
A tower, packed with Montz B1-200 metal structured packing, is to be designed to absorb SO2 from air by scrubbing with water. The entering gas, at an SO2-free flow rate of 6.90 lbmollh-ft2 of bed cross section, contains 80 mol% air and 20 mol% SO2. Water enters at a flow rate of 364 lbmol/h-ft2 of bed cross section. The exiting gas is to contain only 0.5 mol% SO2. Assume that neither air nor water will be transferred between phases and that the tower operates at 2 atm and 30°C. Equilibrium data in mole fractions for SO2 solubility in water at 30°C and 2 atm (Perry's Chemical Engineers Handbook, 4th ed., Table 14.31, p. 14-6) have been fitted by a least-squares method to the equation
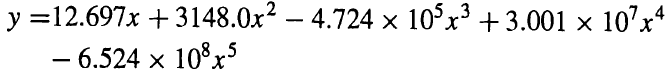
(a) Derive the following molar material balance operating line for SO2 mole fractions:
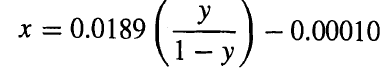
(b) Write a computer program or use a spreadsheet program to calculate the number of required transfer units based on the overall gas-phaseresistance.
4.724 x 10x +3.001 10'x* .4 .2 y =12.697x +3148.0x? - 6.524 x 10%x5
Step by Step Solution
3.39 Rating (165 Votes )
There are 3 Steps involved in it
a A material balance for the solute SO 2 from the top of the column to an intermediate point gives S... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (244).docx
120 KBs Word File


