Question: Determine the diameter and packed height of a countercurrently operated packed tower required to recover 99% of the ammonia from a gas mixture that contains
Determine the diameter and packed height of a countercurrently operated packed tower required to recover 99% of the ammonia from a gas mixture that contains 6 mol% NH3 in air. The tower, packed with l-in. metal Pall rings, must handle 2,000 ft3/min of gas as measured at 68oF and 1 atm. The entering water-absorbent rate will be twice the theoretical minimum, and the gas velocity will be such that it is 50% of the flooding velocity. Assume isothermal operation at 68°F and 1 atm. Equilibrium data are given in Figure 6.49
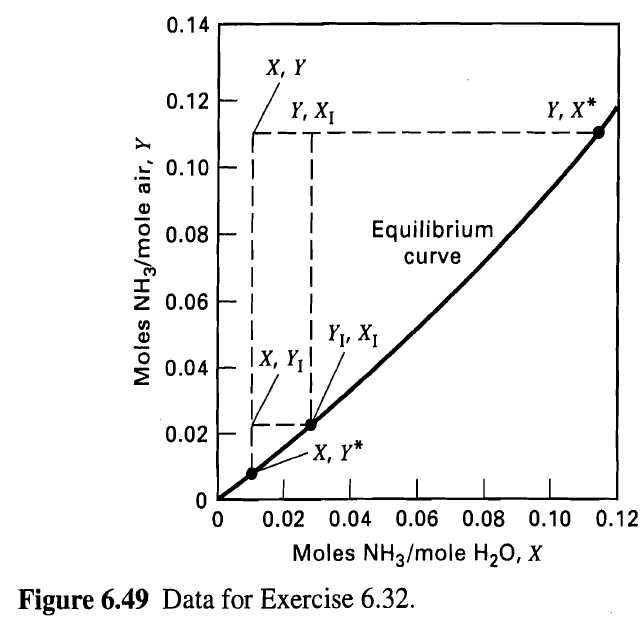
0.14 X, Y 0.12 Y, X* Y, X1 0.10 | Equilibrium 0.08 curve 0.06 EI Y1, X1 |X, Y! 0.04 0.02 -, * 0.02 0.04 0.06 0.08 0.10 0.12 Moles NH3/mole H20, X Figure 6.49 Data for Exercise 6.32. Moles NH3/mole air, Y
Step by Step Solution
3.48 Rating (171 Votes )
There are 3 Steps involved in it
Entering gas rate is Compute material balance NH 3 in entering gas 006312 1872 lbmolh NH 3 in exiting gas 0011872 019 lbmolh Air in entering and exiti... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (243).docx
120 KBs Word File


