Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I attached my three incorrect attempts Read Section 11.6. You can click on the Review link to access the section in your eText. Assuming that
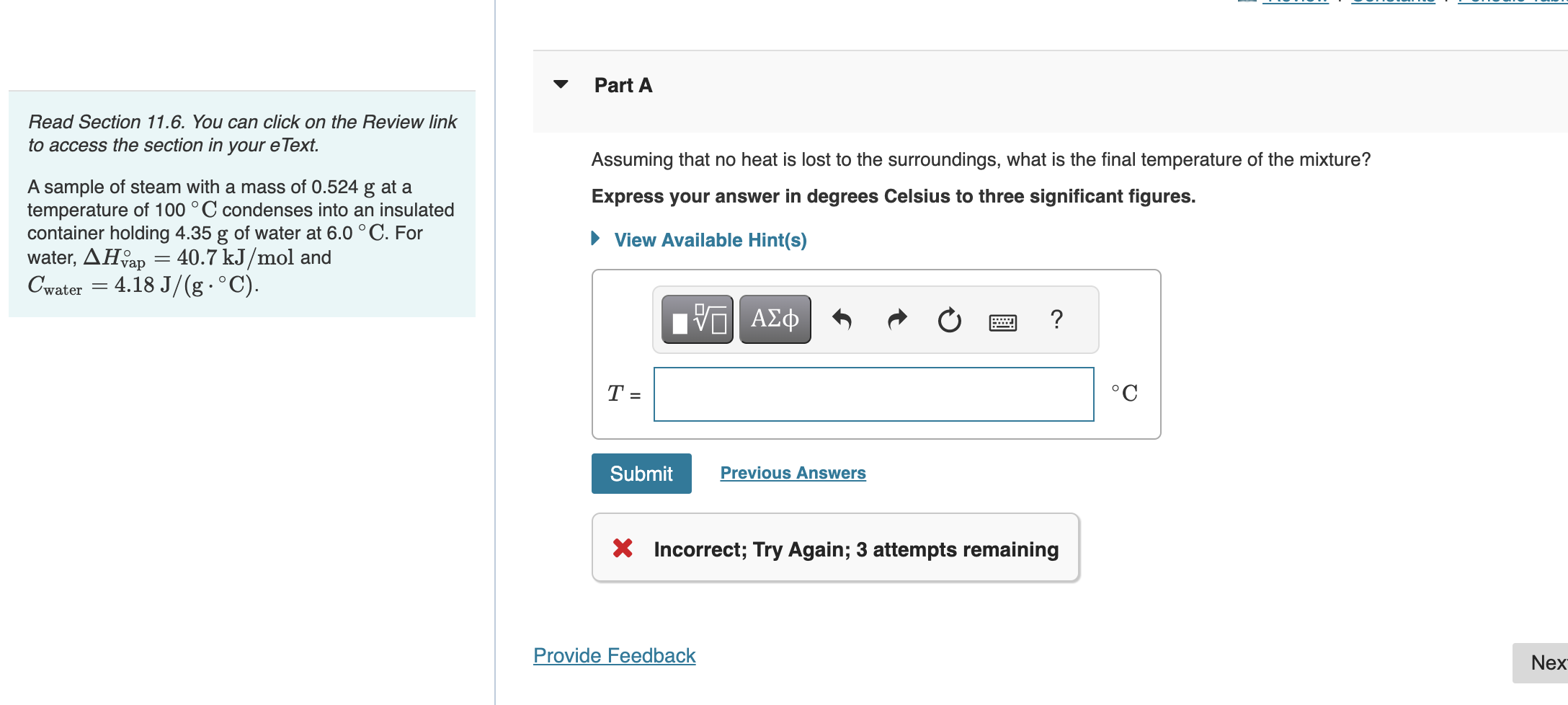

I attached my three incorrect attempts
Read Section 11.6. You can click on the Review link to access the section in your eText. Assuming that no heat is lost to the surroundings, what is the final temperature of the mixture? A sample of steam with a mass of 0.524g at a temperature of 100C condenses into an insulated Express your answer in degrees Celsius to three significant figures. container holding 4.35g of water at 6.0C. For water, Hvap=40.7kJ/mol and Cwater=4.18J/(gC) Incorrect; Try Again; 3 attempts remaining Submitted Answers ANSWER 1: ANSWER 2: Use the relation q=mcT where m is the mass in grams, c is the specific heat capacity, and T is the change in ternperature. The energy required to condense and cool the steam, qutesm, has two terms: the energy requ energy required to then cool it to the linal temperature. qwater is the energy required to heat the water. Substituling this into the equation 0=qwaterqsteam gives 0=mwatercwater(TfinalTinitial,water)+msteancwater(TfinalTinitial,ateam)+18.016g/mol(mtansm)(Hveq) Rearrange this equation to solve for Tfinal. ANSWER 3: Use the relation q=mcT where m is the mass in grams, c is the specific heat capacity, and T is the change in ternperature. The energy required to condense and cool the steam, q atesm, has two terms: the energy requ energy required to then cool it to the final temperature. qxatcr is the energy required to heat the water. Substituting this into the equation 0=qxaterqsteam gives 0=mwatercwater(TfinalTinitial,water)+msteancwater(TfinalTinitial,stcam)+18.016g/mol(mstasm)(Hvaq) Rearrange this equation to solve for TfinalStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


