Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I believe the value of n for the HOMO of molecule B is 5 if they can be verified. Im a bit stumped on how
 I believe the value of "n" for the HOMO of molecule B is 5 if they can be verified. Im a bit stumped on how to find the value of "L" if a is the length of the bond. How would you find that?
I believe the value of "n" for the HOMO of molecule B is 5 if they can be verified. Im a bit stumped on how to find the value of "L" if a is the length of the bond. How would you find that?
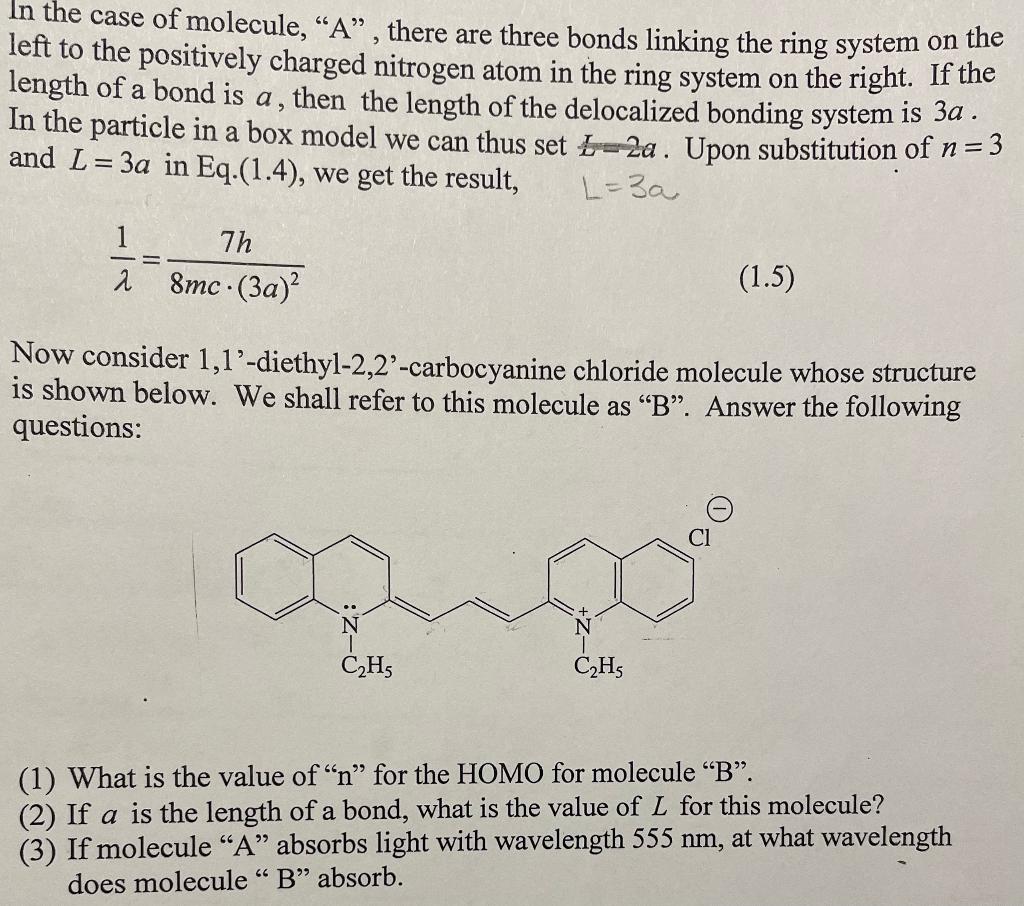
In the case of molecule, "A", there are three bonds linking the ring system on the left to the positively charged nitrogen atom in the ring system on the right. If the length of a bond is a, then the length of the delocalized bonding system is 3a. In the particle in a box model we can thus set t=2a. Upon substitution of n=3 and L=3a in Eq.(1.4), we get the result, L=3a 1=8mc(3a)27h Now consider 1,1'-diethyl-2,2'-carbocyanine chloride molecule whose structure is shown below. We shall refer to this molecule as " B ". Answer the following questions: (1) What is the value of " n " for the HOMO for molecule "B". (2) If a is the length of a bond, what is the value of L for this molecule? (3) If molecule " A " absorbs light with wavelength 555nm, at what wavelength does molecule " B " absorb
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


