Question
I don't know which one I got wrong (a) Given the following reactions and their heats of combustion, calculate the heat of combustion per CH
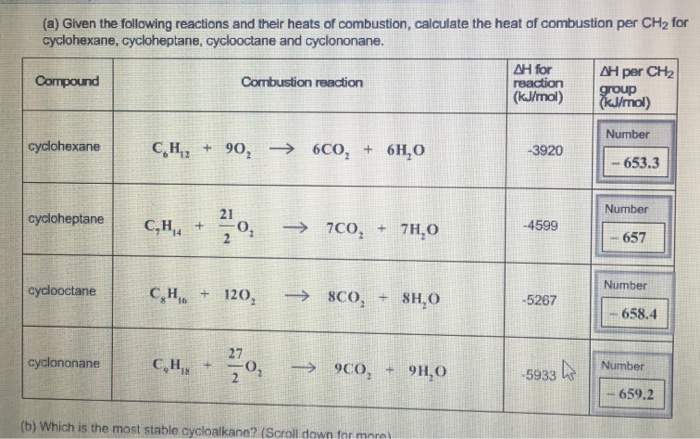
(a) Given the following reactions and their heats of combustion, calculate the heat of combustion per CH for cyclohexane, cycloheptane, cyclooctane and cyclononane. Compound cyclohexane cycloheptane cyclooctane cyclononane Combustion reaction CH, + 90, ) 6CO, + 6H,O 21 CH4 +0, CH + 120, C,H,, + 0, 7CO 7CO + 7HO SCO, + SHO 9CO, + 9H0 (b) Which is the most stable cycloalkane? (Scroll down for more AH for reaction (kJ/mol) -3920 -4599 -5267 -5933 AH per CH group (kJ/mol) Number -653.3 Number 657 Number 658.4 Number 659.2
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To calculate ring strain in cyclic system angle strain and heat of combustions are used More the ang...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Biochemistry Concepts and Connections
Authors: Dean R. Appling, Spencer J. Anthony Cahill, Christopher K. Mathews
1st edition
321839927, 978-0133853490, 133853497, 978-0321839923
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



