Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i keep getting 99.694 lbm/hr but the math doesnt add up for the rest the system, if you get 99.694 can you show me the
i keep getting 99.694 lbm/hr but the math doesnt add up for the rest the system, if you get 99.694 can you show me the math for the rest of the system? 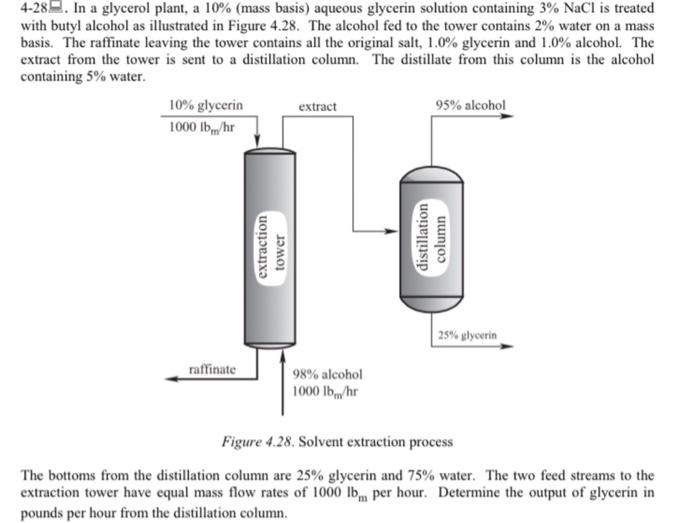
4-28. In a glycerol plant, a 10% (mass basis) aqueous glycerin solution containing 3% NaCl is treated with butyl alcohol as illustrated in Figure 4.28. The alcohol fed to the tower contains 2% water on a mass basis. The raffinate leaving the tower contains all the original salt, 1.0% glycerin and 1.0% alcohol. The extract from the tower is sent to a distillation column. The distillate from this column is the alcohol containing 5% water. 10% glycerin extract 95% alcohol 1000 lb/hr extraction tower distillation column 25% glycerin raffinate 98% alcohol 1000 lb/hr Figure 4.28. Solvent extraction process The bottoms from the distillation column are 25% glycerin and 75% water. The two feed streams to the extraction tower have equal mass flow rates of 1000 lb. per hour. Determine the output of glycerin in pounds per hour from the distillation column 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


