Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I know the rate law is r = k[OH-][CH3COOC6H5], but I can't figure out part b. Could you please use a graph? Thank you for
I know the rate law is r = k[OH-][CH3COOC6H5], but I can't figure out part b. Could you please use a graph? Thank you for your help.
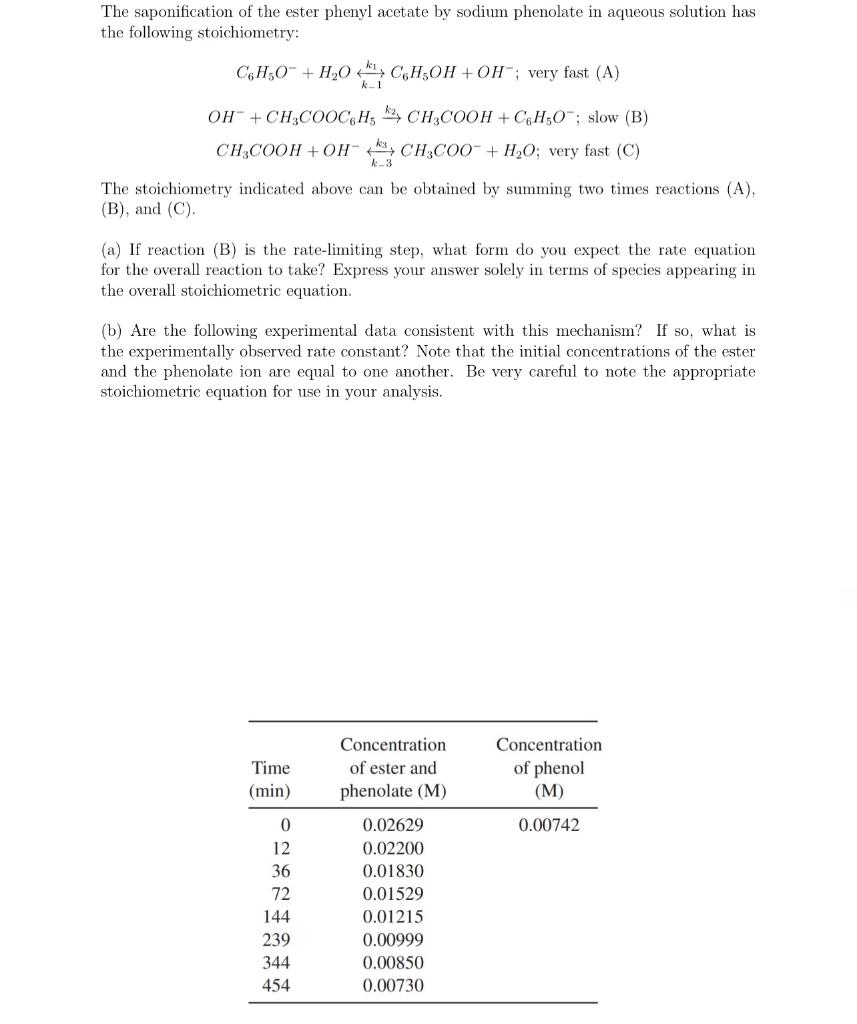
The saponification of the ester phenyl acetate by sodium phenolate in aqueous solution has the following stoichiometry: C6H5O+H2Ok1k1C6H5OH+OH;veryfast(A)OH+CH3COOC6H5k2CH3COOH+C6H5O;slow(B)CH3COOH+OHk3k3CH3COO+H2OOveryfast(C) The stoichiometry indicated above can be obtained by summing two times reactions (A), (B), and (C). (a) If reaction (B) is the rate-limiting step, what form do you expect the rate equation for the overall reaction to take? Express your answer solely in terms of species appearing in the overall stoichiometric equation. (b) Are the following experimental data consistent with this mechanism? If so, what is the experimentally observed rate constant? Note that the initial concentrations of the ester and the phenolate ion are equal to one another. Be very careful to note the appropriate stoichiometric equation for use in your analysis
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


