Question
I need help answering the prelab questions: Pre-Lab Questions: 1. Establish a single equation that will allow you to calculate the molar mass from the
I need help answering the prelab questions:
Pre-Lab Questions:
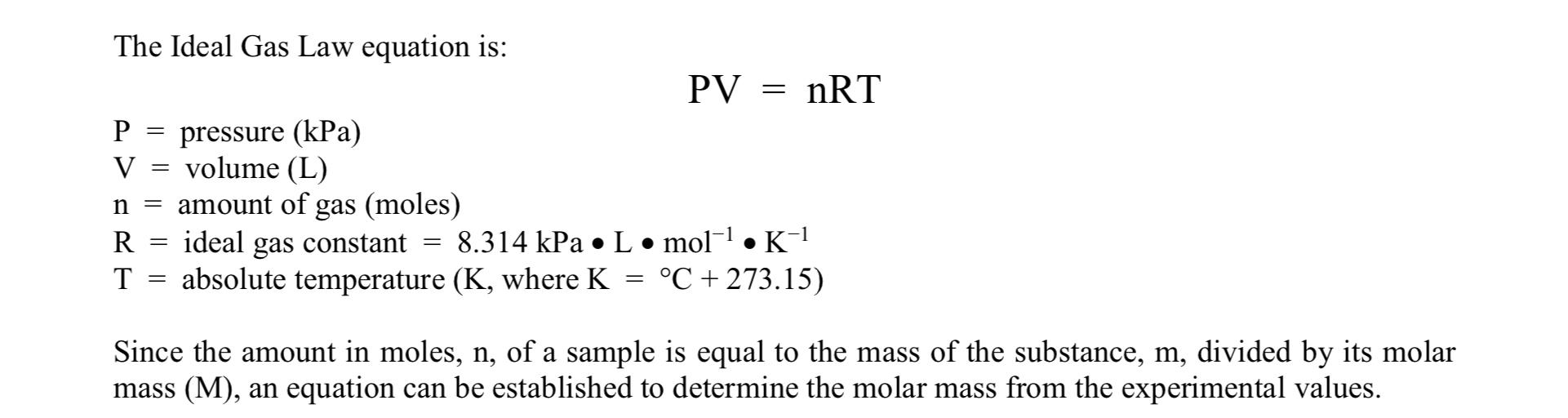
1. Establish a single equation that will allow you to calculate the molar mass from the measurements conducted in this experiment.
2. How is the pressure inside the flask determined in this experiment? Fully explain.
3. Explain why the mass of liquid before heating is of little use in determining the molar mass?
4. At the end of the experiment, the mass of liquid is 0.200g at 101.00 kPa in a 125mL flask at room temperature (25C), what is its molar mass?
5. If the unknown assigned to you is methanol (CH3OH), what mass of it would be present in the 125-mL Erlenmeyer when it is removed from the hot water beaker?
6. Initially 8.0 mL of methanol (density = 0.791 g/mL) were introduced in the Erlenmeyer. Considering the result obtained in question 5, explain what happened.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


