Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help apply my lab results to calculations. I made the solutions up to exactly 100.0 mL each using deionized water. 2. Present the
I need help apply my lab results to calculations. 



I made the solutions up to exactly 100.0 mL each using deionized water.
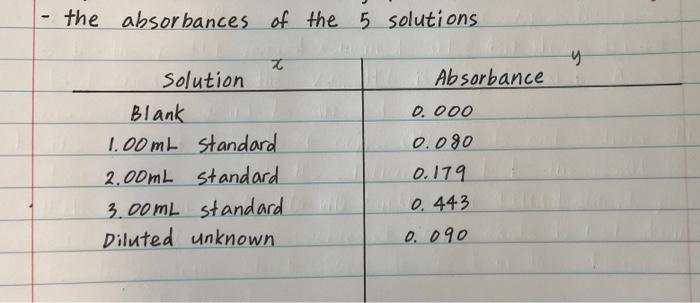
2. Present the concentration of iron(II)ions (Fe) in mg/L and absorbance of the blank and standards in a table for graphing. 3. Construct a calibration graph from the above values and draw the best-fitting straight line through the data points. Determine the concentration of iron with its unit in the diluted unknown solution on the calibration graph. From the diluted unknown concentration, calculate the concentration of iron in the original unknown solution. 5. Calculate the mass of iron in the solid unknown sample. 4. the absorbances of the 5 solutions y x Solution Blank 1.00mL standard 2.00mL standard 3.00mL standard Diluted unknown Ab sorbance 0.000 0.080 0.179 0.443 0.090 Preparation of standard Solutions the concentration of the Fe 2+ stock solution 52.18 mg / m2 L Preparation of the unknown section - the tared mass of K Fe (C204) 3 H2O 0.2119 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


