Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The molar mass of OH is 17.008 g mol Please complete the following table. For each of your trials, calculate the mmol HCI neutralized
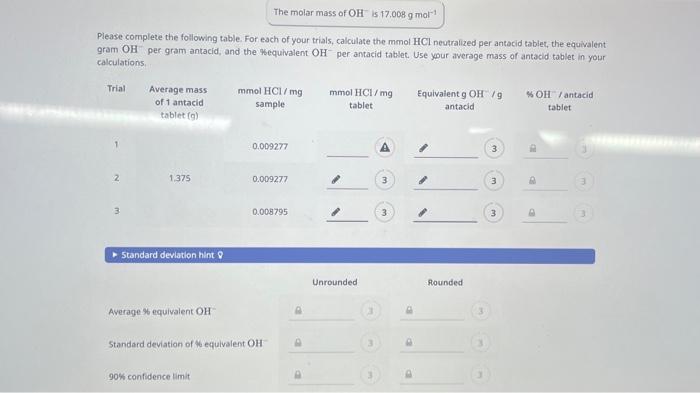
The molar mass of OH is 17.008 g mol Please complete the following table. For each of your trials, calculate the mmol HCI neutralized per antacid tablet, the equivalent gram OH per gram antacid, and the %equivalent OH per antacid tablet. Use your average mass of antacid tablet in your calculations. Trial Average mass of 1 antacid mmol HCl/mg sample mmol HCl/mg tablet Equivalent g OH/9 antacid % OH antacid tablet tablet (g) 0.009277 2 1.375 0.009277 3 Standard deviation hint 0.008795 Average % equivalent OH Standard deviation of % equivalent OH 90% confidence limit Unrounded Rounded 3 3
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To complete the table follow these calculations step by step Step 1 Calculate mmol HCl neutralized per mg tablet The mmol HCl neutralized per mg table...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


