Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I NEED HELP STARTING FROM D PLEASE! has the form K=(qCO(gas)/N)qCO(bound), where qCO(gas)=qtransqrotqvibqelec. Once bound to the heme group the CO molecule is immobilized and

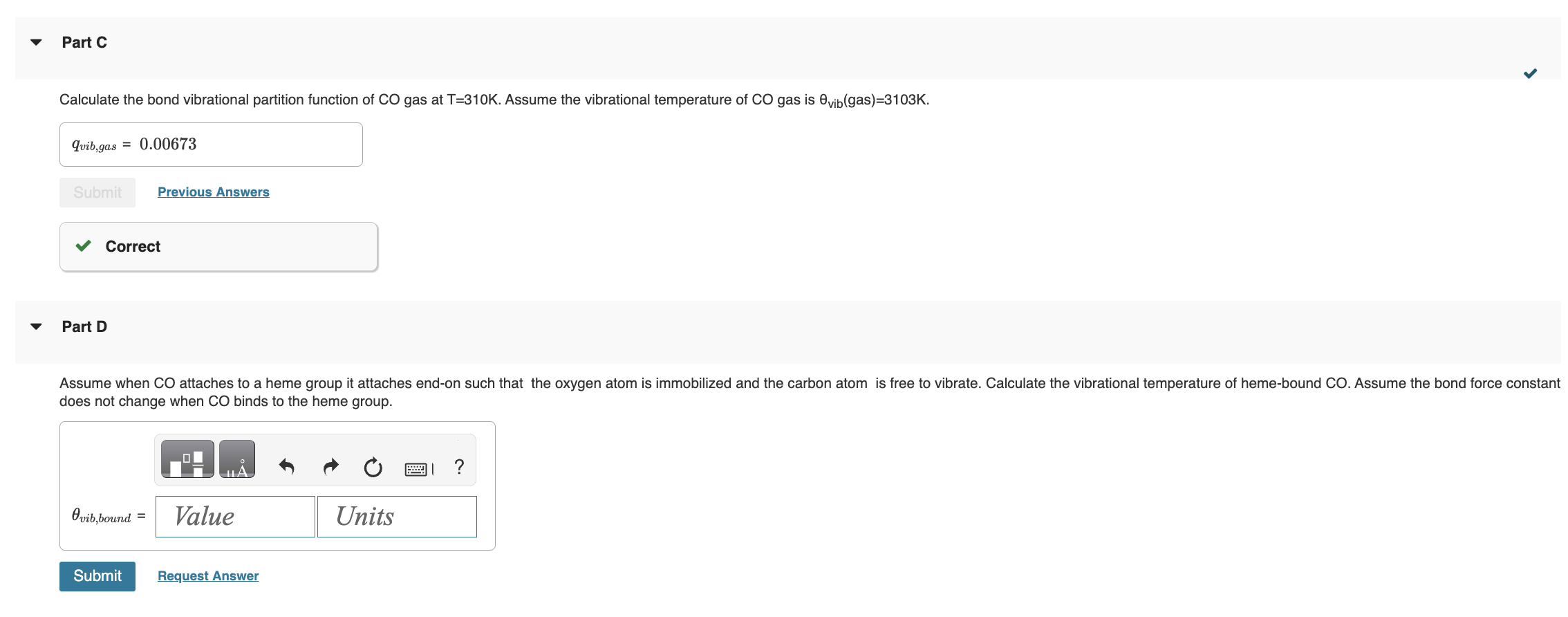
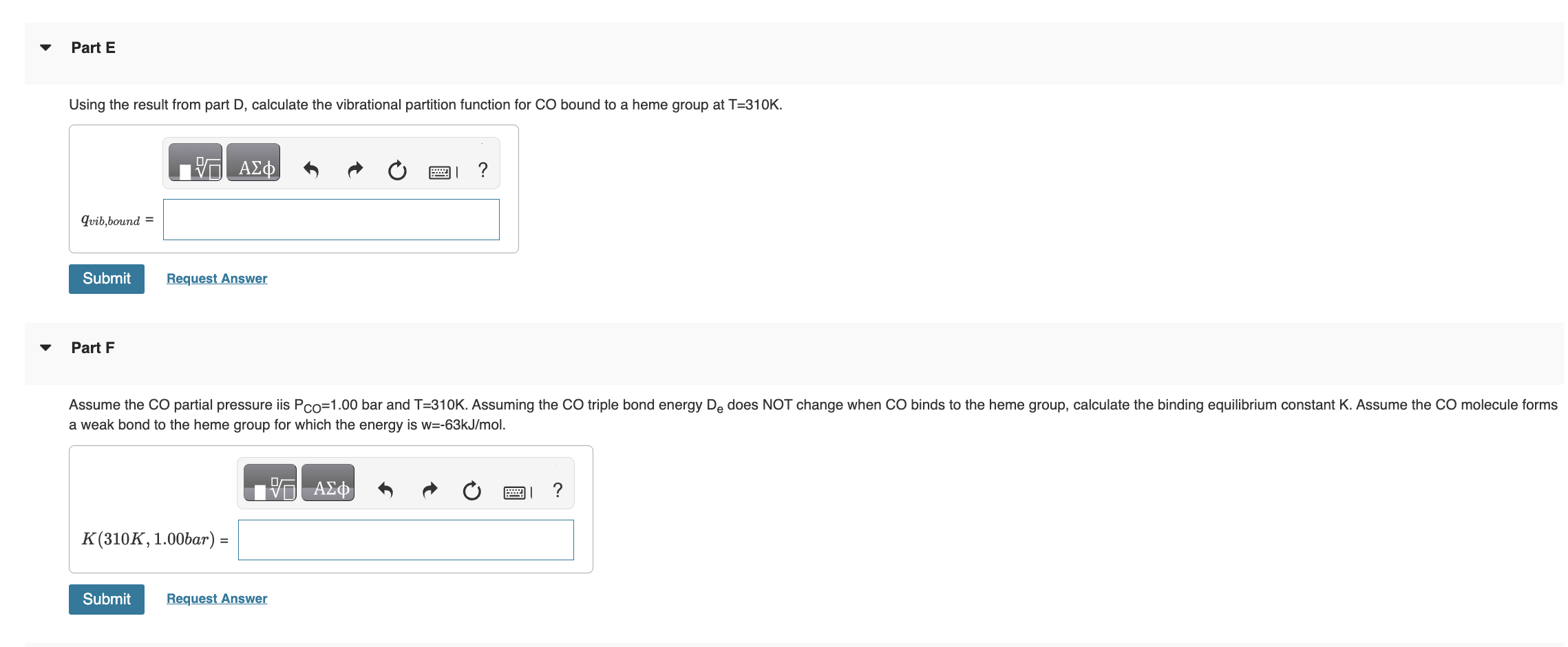
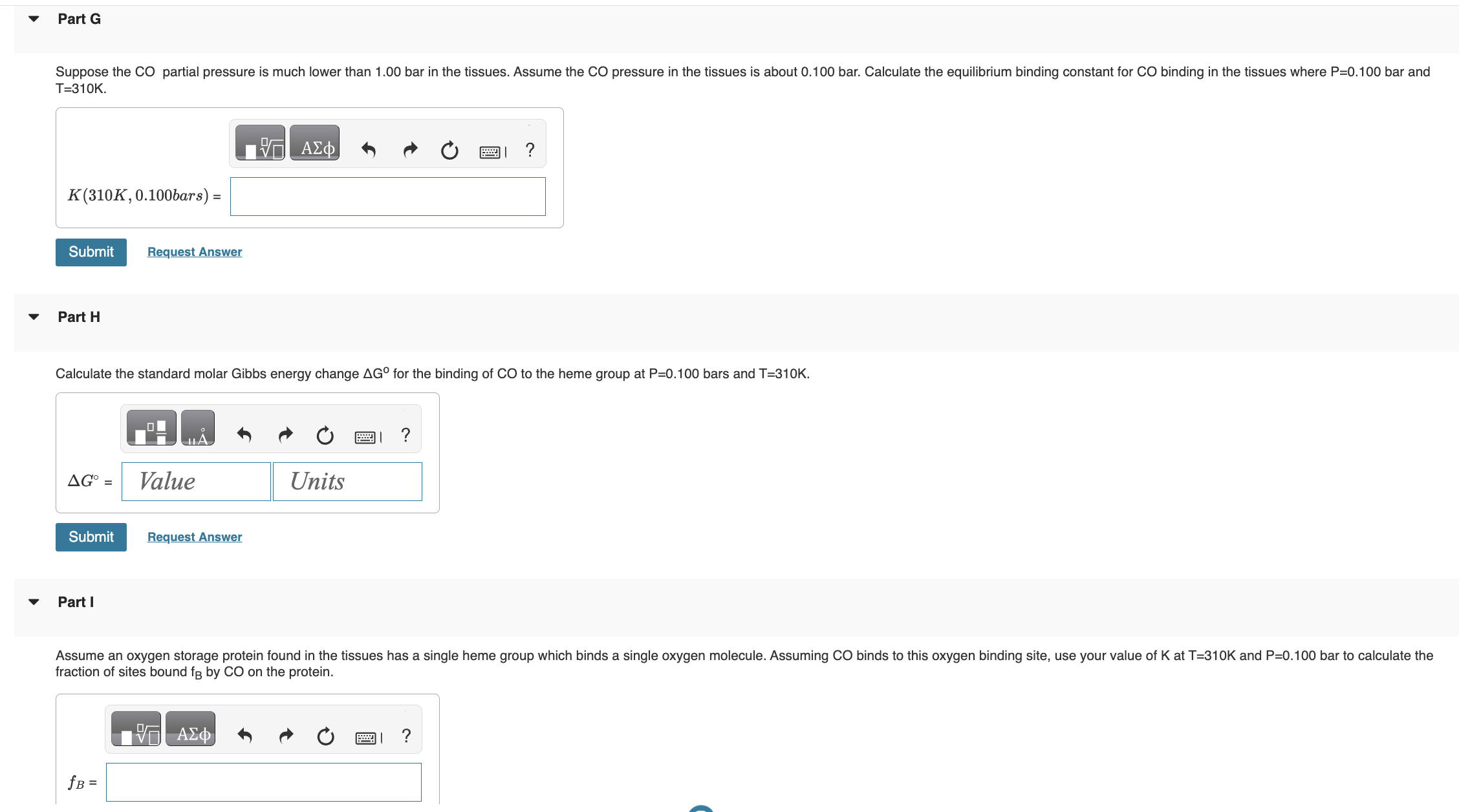
I NEED HELP STARTING FROM D PLEASE!
has the form K=(qCO(gas)/N)qCO(bound), where qCO(gas)=qtransqrotqvibqelec. Once bound to the heme group the CO molecule is immobilized and no longer translates or rotates. Therefore we assume CO molecule is localized on the heme group and distinguishable. Give all answers to three significant figures. Part A Calculate the thermal wavelength (also called the deBoglie wavelength) CO for CO at T=310K. CO=1.881011m Part B Calculate the rotational partition function of CO at T=310K. . Assume the rotational temperature of CO is rot=2.77K. Calculate the bond vibrational partition function of CO gas at T=310K. Assume the vibrational temperature of CO gas is vib (gas) =3103K. Part D does not change when CO binds to the heme group. Using the result from part D, calculate the vibrational partition function for CO bound to a heme group at T=310K. Calculate the standard molar Gibbs energy change G for the binding of CO to the heme group at P=0.100 bars and T=310K. Part I fraction of sites bound fB by CO on the proteinStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


