Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i need help understanding Aluminum oxide (used as an adsorbent or a catalyst for organic reactions) forms when aluminum reacts with oxygen. 4A(s)+3O2(g)2Al2O3(s) A muxture
i need help understanding 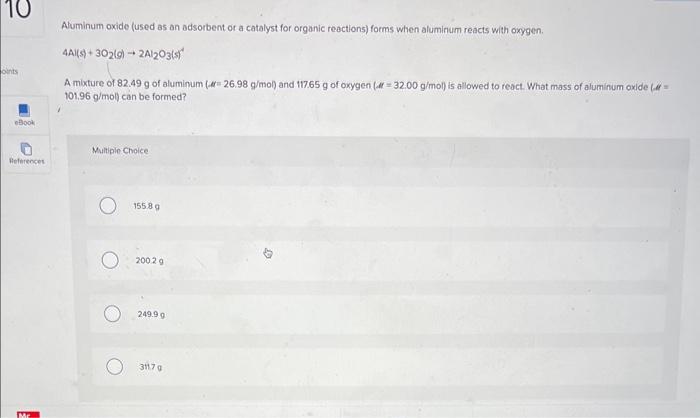
Aluminum oxide (used as an adsorbent or a catalyst for organic reactions) forms when aluminum reacts with oxygen. 4A(s)+3O2(g)2Al2O3(s) A muxture of 82.49g of aluminum (.e= 26.98g/mol) and 117.65g of oxygen (if=32.00g/mol) is allowed to react. What mass of aluminum oxide (if = 101.96g/mol ) can be formed? Nultipio Choice 15589 20029 249.99 3170 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


