Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need help with these problems 13. When 45.0 grams of butane (C4H10) is burned in the presence of 45.0 grams of oxygen, 10.5 grams
I need help with these problems 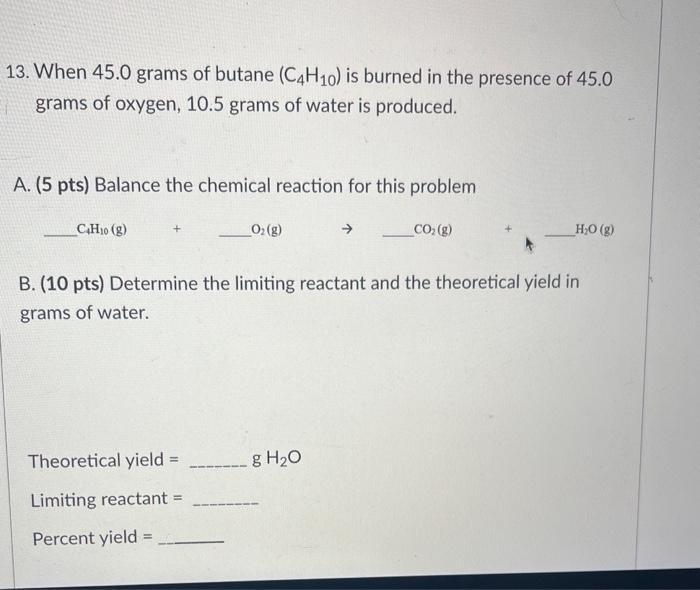

13. When 45.0 grams of butane (C4H10) is burned in the presence of 45.0 grams of oxygen, 10.5 grams of water is produced. A. (5 pts) Balance the chemical reaction for this problem C4H10(g)+O2(g)+CO2(g)+H2O(g) B. (10 pts) Determine the limiting reactant and the theoretical yield in grams of water. Theoretical yield = gH2O 11. (10 pts) Find the empirical formula for a compound containing 61.31% carbon, 5.14% hydrogen, 10.21% nitrogen, and 23.33% oxygen. Be sure to type the subscripts you find for C,H,N, and O into the spaces below. 12. When gaseous carbon monoxide reacts with oxygen gas, carbon dioxide gas is produced. A. (5 pts) Finish writing the chemical reaction by adding the remaining chemical formulas of the two reactants, their physical states, and any coefficients needed to properly balance the chemical reaction. B. (5 pts) What type of reaction is this classified as: D. (5 pts) How many molecules of carbon dioxide are made when 56g of CO are used in the balance reaction in Part A 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


