Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i need help with this question please help. please show step by step solution to this problem. thankyou question: Data: background infomation: sample solution: Using
i need help with this question please help. please show step by step solution to this problem. thankyou 
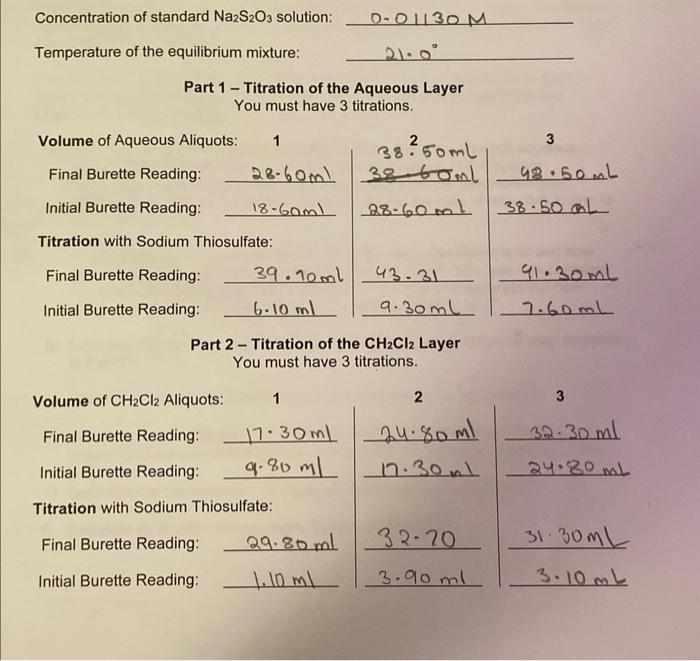
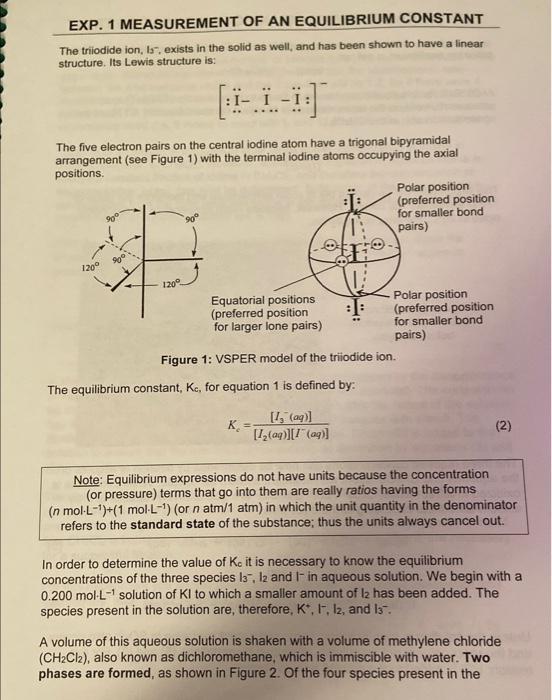


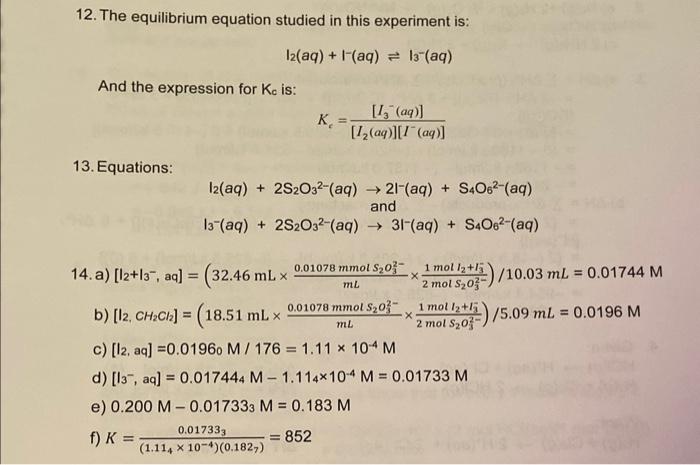
Using your own lab-obtained data, what was your calculated Kc in water? Report your answer to the nearest whole number. Concentration of standard Na2S2O3 solution: 0.01130M Temperature of the equilibrium mixture: 210 Part 1 - Titration of the Aqueous Layer You must have 3 titrations. Part 2 - Titration of the CH2Cl2 Layer You must have 3 titrations. The triodide ion, I5, exists in the solid as well, and has been shown to have a linear structure. Its Lewis structure is: [:III:] The five electron pairs on the central iodine atom have a trigonal bipyramidal arrangement (see Figure 1) with the terminal iodine atoms occupying the axial positions. Figure 1: VSPER model of the triiodide ion. The equilibrium constant, Kc, for equation 1 is defined by: Kc=[I2(aq)][I(aq)][I3(aq)] Note: Equilibrium expressions do not have units because the concentration (or pressure) terms that go into them are really ratios having the forms (nmolL1)+(1molL1) (or natm/1atm) in which the unit quantity in the denominator refers to the standard state of the substance; thus the units always cancel out. In order to determine the value of K0 it is necessary to know the equilibrium concentrations of the three species l3,l2 and Iin aqueous solution. We begin with a 0.200molL1 solution of KI to which a smaller amount of I2 has been added. The species present in the solution are, therefore, K+,I,I2, and I3. A volume of this aqueous solution is shaken with a volume of methylene chloride (CH2Cl2), also known as dichloromethane, which is immiscible with water. Two phases are formed, as shown in Figure 2. Of the four species present in the EXP. 1 MEASUREMENI aqueous layer, only l2 is appreciably soluble in CH2Cl2 and after some swirling, the following equilibrium is set up: I2(aq)I2(CH2Cl2) At 25C, K for this equilibrium has been found to have a value of 176. That is, the concentration of l2 in the aqueous layer is always a constant fraction, 1/176, of the concentration of I2 in the CH2Cl2. K=[I2(aq)][H2(CH2C2)]=176at25C This experiment will be carried out at room temperature and therefore the value of 176 is technically not correct. However, because room temperature is reasonably close to 25C, the error is negligible. Figure 2: Equilibria in the two-phase system before analysis. The three required concentrations can now be determined by titration of aliquots of the aqueous and CH2Cl2 layers with standard sodium thiosulfate. While thiosulfate is oxidized to tetrathionate, S4O62, both l2 and I3 - are quantitatively reduced to Iby sodium thiosulfate according to the equations: I2(aq)+2S2O32(aq)2I(aq)+S4O62(aq)andI3(aq)+2S2O32(aq)3I(aq)+S4O62(aq) Unless the solution is slightly acidic, some concurrent oxidation to SO42 also occurs and the reactions are not completely described by equations (5) and (6). For this reason, HCl is added to the analyte prior to titration. Titration of an aliquot of the aqueous layer leads to the total concentration of reducible iodine, namely the total number of triiodide ions plus iodine molecules in the water layer. Based on equations (5) and (6), we see that the stoichiometric ratio between the total reducible iodine and the thiosulfate will be 1:2. So if there were 4 moles of l2(aq) and 6 moles of I3(aq) present in the aliquot, then the total reducible iodine would be 10 moles, and 20 moles of thiosulfate would be required to reach the equivalence point (i.e. 8 moles to react with l2 and 12 moles for the reaction with I3 ). Titration of an aliquot of the CH2Cl2 layer leads to the concentration of iodine in dichloromethane, l2(CH2Cl2). However, according to Equation 4, aqueous iodine is always a constant fraction of iodine in CH2Cl2, and therefore the concentration of aqueous iodine, l2(aq), can now be determined. Subtraction of the aqueous iodine concentration from the total reducible iodine in the aqueous phase leads to the concentration of aqueous triiodide ion, I3(aq). Only the concentration of aqueous iodide remains to be determined. Since the original aqueous solution was prepared by adding l2 to 0.200molL1 aqueous potassium iodide, we know that: [K+](aq)=[1](aq)+[1](aq)=0.200molL1 You should prove for yourself that this is true, using equation (1). It follows that: [1](aq)=0.200molL1[l](aq) In the titration of the aqueous aliquot the endpoint is recognized by the disappearance of the brown colour of the I2 and I3 - solution ( I3 alone is colourless). The endpoint can be made much clearer by adding some starch solution after the colour of the solution has nearly faded. 133 forms an intensely blue-coloured complex with starch. The titration is then continued until the blue colour vanishes. Dilute HCl is added to the solution to ensure that the reactions occur entirely as described by equations (5) and (6). The endpoint in the titration of the CH2Cl2 aliquot is clearly indicated by the disappearance of the purple colour, and no starch is necessary. However, vigorous and continuous agitation of the contents of the flask to ensure mixing (keep in mind that CH2Cl2 is immiscible with water), and slow addition of the titrant, are necessary for a successful titration. For this experiment you should revisit the sections on "Volumetric Glassware and Techniques" and "Titrations" in the introduction to the lab manual. 12. The equilibrium equation studied in this experiment is: I2(aq)+I(aq)I3(aq) And the expression for Kc is: Kc=[I2(aq)][I(aq)][I3(aq)] 13. Equations: I2(aq)+2S2O32(aq)2I(aq)+S4O62(aq)I3(aq)+2S2O32(aq)31(aq)+S4O62(aq) b) [I2,CH2Cl2]=(18.51mLmL0.01078mmolS2O322molS2O321moll2+l3)/5.09mL=0.0196M c) [I2,aq]=0.01960M/176=1.11104M d) [I3,aq]=0.017444M1.114104M=0.01733M e) 0.200M0.017333M=0.183M f) K=(1.114104)(0.1827)0.017333=852 question:

Data:

background infomation:



sample solution:

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


