Answered step by step
Verified Expert Solution
Question
1 Approved Answer
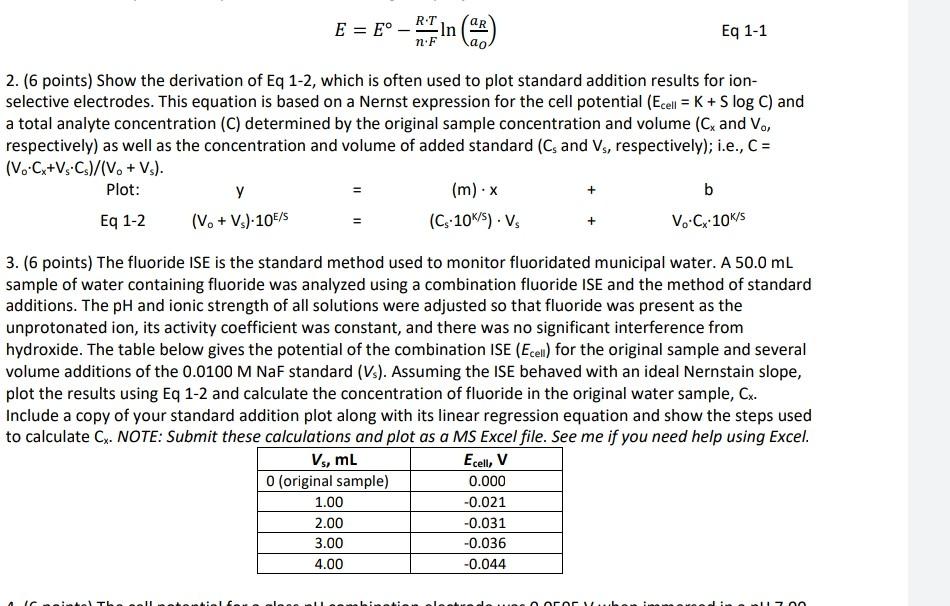
I need it urgently E=EnFRTln(a0aR) 2. (6 points) Show the derivation of Eq 1-2, which is often used to plot standard addition results for ionselective
I need it urgently
E=EnFRTln(a0aR) 2. (6 points) Show the derivation of Eq 1-2, which is often used to plot standard addition results for ionselective electrodes. This equation is based on a Nernst expression for the cell potential (Ecell=K+SlogC) and a total analyte concentration (C) determined by the original sample concentration and volume (Cx and V0, respectively) as well as the concentration and volume of added standard (Cs and Vs, respectively); i.e., C= (V0Cx+VsCs)/(V0+Vs) Plot:Eq1-2y(V0+Vs)10E/s==(m)x(C510k/s)Vs++bV0Cx10k/s 3. (6 points) The fluoride ISE is the standard method used to monitor fluoridated municipal water. A 50.0mL sample of water containing fluoride was analyzed using a combination fluoride ISE and the method of standard additions. The pH and ionic strength of all solutions were adjusted so that fluoride was present as the unprotonated ion, its activity coefficient was constant, and there was no significant interference from hydroxide. The table below gives the potential of the combination ISE ( Ecell ) for the original sample and several volume additions of the 0.0100MNaF standard (Vs). Assuming the ISE behaved with an ideal Nernstain slope, plot the results using Eq 1-2 and calculate the concentration of fluoride in the original water sample, Cx. Include a copy of your standard addition plot along with its linear regression equation and show the steps used to calculate Cx. NOTE: Submit these calculations and plot as a MS Excel file. See me if you need help using ExcelStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


