Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I need the answer for this question pleas. A crystal of chalcanthite (CuSO45H2O) dissolves in a large tank of pure water according to the following
I need the answer for this question pleas.
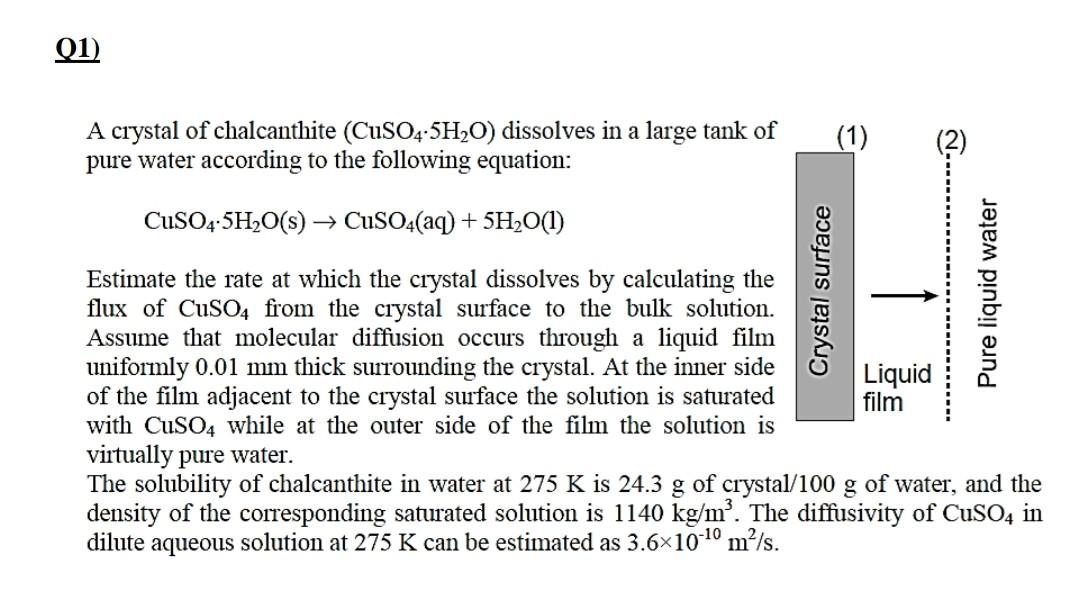
A crystal of chalcanthite (CuSO45H2O) dissolves in a large tank of pure water according to the following equation: CuSO45H2O(s)CuSO4(aq)+5H2O(l) Estimate the rate at which the crystal dissolves by calculating the flux of CuSO4 from the crystal surface to the bulk solution. Assume that molecular diffusion occurs through a liquid film uniformly 0.01mm thick surrounding the crystal. At the inner side of the film adjacent to the crystal surface the solution is saturated with CuSO4 while at the outer side of the film the solution is virtually pure water. The solubility of chalcanthite in water at 275K is 24.3g of crystal/100 g of water, and the density of the corresponding saturated solution is 1140kg/m3. The diffusivity of CuSO4 in dilute aqueous solution at 275K can be estimated as 3.61010m2/sStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


