Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I really need help quickly! I will rate! Please include units. Chemists often use molarity M, in moles/liter, to measure the concentration of solutions. Molarity
I really need help quickly! I will rate! Please include units. 
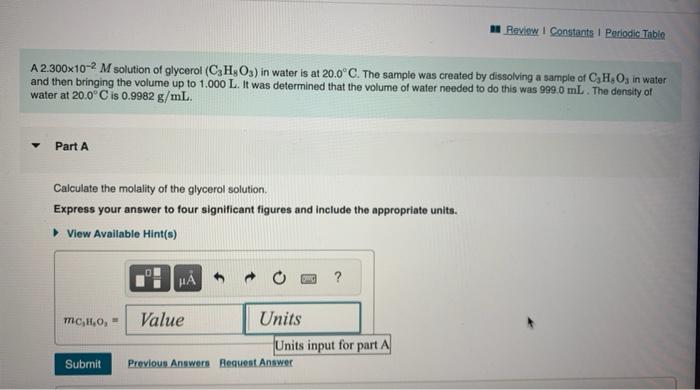
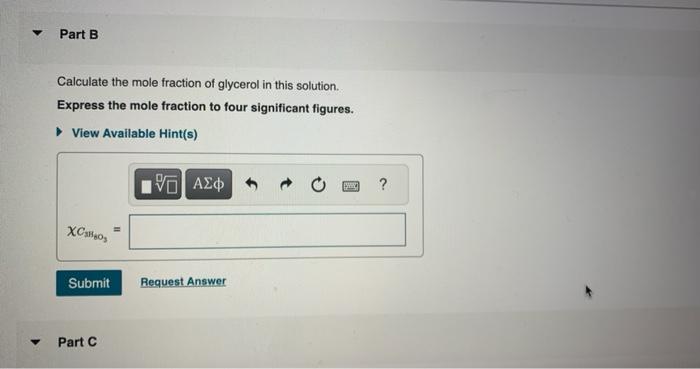
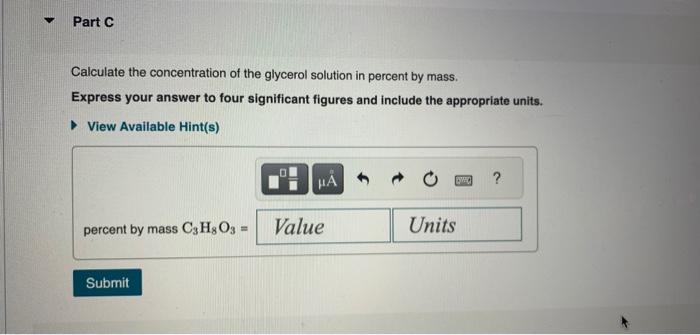
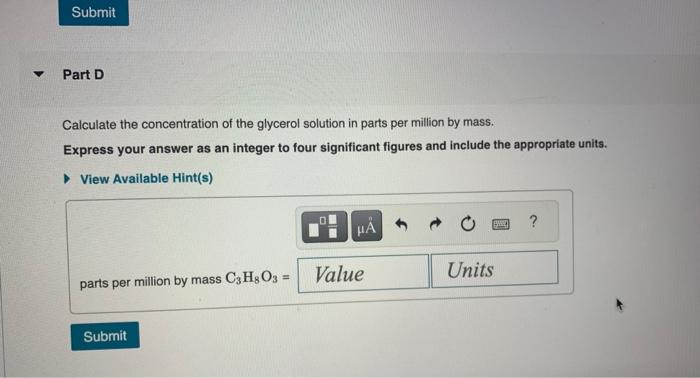
Chemists often use molarity M, in moles/liter, to measure the concentration of solutions. Molarity is a common unit of concentration because the volume of a liquid is very easy to measure. However, the drawback of using molarity is that volume is a temperature-dependent quantity. As temperature changes, density changes, which affects volume. Volume markings for most laboratory glassware are calibrated for room temperature, about 20C. Fortunately, there are several other ways of expressing concentration that do not involve volume and are therefore temperature independent. and then bringing the volume up to 1.000L. It was determined that the volume of water needed to do this was 999.0mL. The density of water at 20.0C is 0.9982g/mL. Part A Calculate the molality of the glycerol solution. Express your answer to four significant figures and include the appropriate units. Calculate the mole fraction of glycerol in this solution. Express the mole fraction to four significant figures. Calculate the concentration of the glycerol solution in percent by mass. Express your answer to four significant figures and include the appropriate units. Calculate the concentration of the glycerol solution in parts per million by mass. Express your answer as an integer to four significant figures and include the appropriate units 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


