Answered step by step
Verified Expert Solution
Question
1 Approved Answer
i. Show that for an ideal solution of A and B, the mole fractions of A in vapor phase in equilibrium with solution is xAv=1+xAl(PBPA1)xBlPBPA
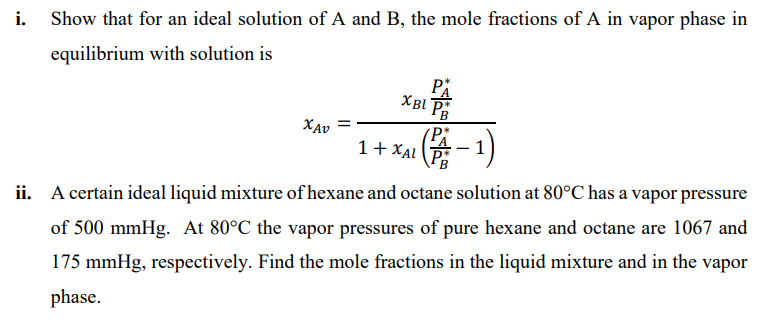 i. Show that for an ideal solution of A and B, the mole fractions of A in vapor phase in equilibrium with solution is xAv=1+xAl(PBPA1)xBlPBPA ii. A certain ideal liquid mixture of hexane and octane solution at 80C has a vapor pressure of 500mmHg. At 80C the vapor pressures of pure hexane and octane are 1067 and 175mmHg, respectively. Find the mole fractions in the liquid mixture and in the vapor phase
i. Show that for an ideal solution of A and B, the mole fractions of A in vapor phase in equilibrium with solution is xAv=1+xAl(PBPA1)xBlPBPA ii. A certain ideal liquid mixture of hexane and octane solution at 80C has a vapor pressure of 500mmHg. At 80C the vapor pressures of pure hexane and octane are 1067 and 175mmHg, respectively. Find the mole fractions in the liquid mixture and in the vapor phase Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


