Answered step by step
Verified Expert Solution
Question
1 Approved Answer
I would like help with question 12. 2 0.10M acetic acid 0.10M sodium 0.10M HCl 0.10M NaOH 1.0M acetic 1.0M sodium acetate distilled water acetate

I would like help with question 12.
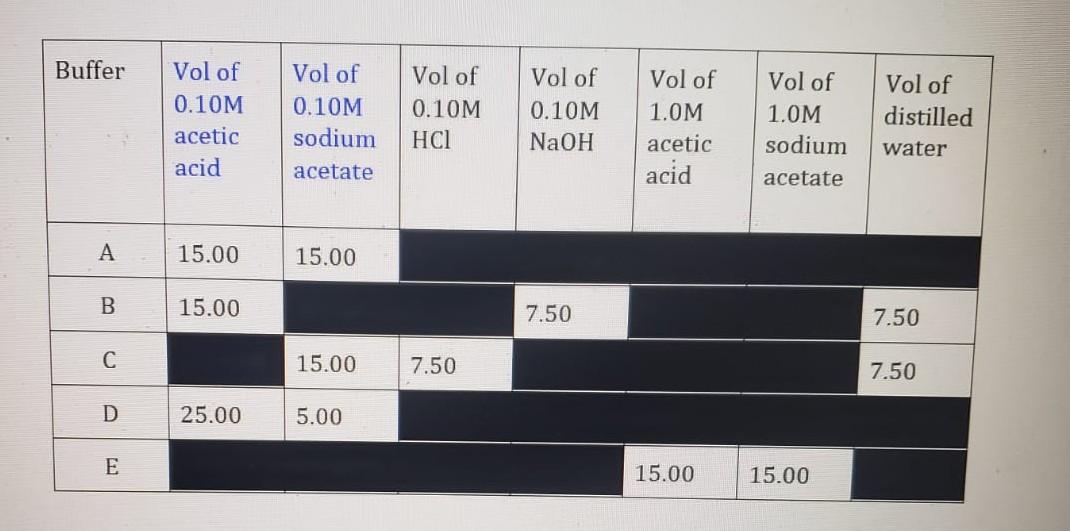
2 0.10M acetic acid 0.10M sodium 0.10M HCl 0.10M NaOH 1.0M acetic 1.0M sodium acetate distilled water acetate acid A 15.00 15.00 CO B 15.00 7.50 7.50 15.00 7.50 7.50 D 25.00 5.00 E 15.00 15.00 8. You will have 30.00mL of each buffer. Record the initial pH of these buffers (A-E) 9. Right click on each of these buffers and "duplicate" the solution. Rename these solutions, so if you are working with buffer E, one of these will be E + acid and the other E + base. 10. Add 2.00mL of 1M HCl to each of the buffer flasks that are labelled "+acid". Record the pH of each buffer after the addition of acid. 11. Add 2.00mL of 1M NaOH to each of the buffer flasks that are labelled "+base". Record the pH of each buffer after the addition of base. 12. Calculate the theoretical initial pH of solutions A and E. Then, calculate the theoretical pH of solution A and solution E after 2 mL of 1.0 M HCl was added to each solution. Don't forget that when the solutions were mixed together, their concentrations changed. You must account for these changes in your calculations. These calculations will be part of your calculations section in your lab report. Buffer Vol of 0.10M acetic acid Vol of 0.10M sodium Vol of 0.10M HCl Vol of 0.10M NaOH Vol of 1.0M acetic acid Vol of 1.0M sodium acetate Vol of distilled water acetate A 15.00 15.00 B 15.00 7.50 7.50 C 15.00 7.50 7.50 D 25.00 5.00 E 15.00 15.00
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


