Question
Use the thermodynamic data in the table to determine the AG for the reaction: H2(g) +Cl2 (g) 2HCl (g) when the partial pressure of
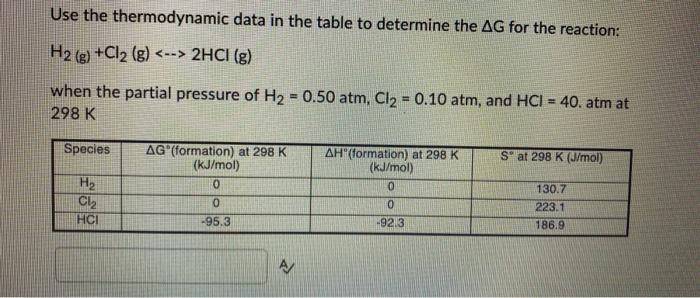
Use the thermodynamic data in the table to determine the AG for the reaction: H2(g) +Cl2 (g) 2HCl (g) when the partial pressure of H = 0.50 atm, Cl = 0.10 atm, and HCI = 40. atm at 298 K Species H Cl HCI AG (formation) at 298 K (kJ/mol) 0 0 -95.3 AH(formation) at 298 K (kJ/mol) 0 0 -92.3 Sat 298 K (J/mol) 130.7 223.1 186.9
Step by Step Solution
3.34 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
the reaction in Hg Cl g Aby for the reaction and Since we ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Contemporary Auditing
Authors: Michael C. Knapp
8th edition
978-0538466790, 538466790, 978-1285066608
Students also viewed these Business Communication questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



