Question
Identify the symbol of the element that corresponds to the following (ground state) electron configurations. Noble Gas Electron Configuration Group Number Period Number 1s2s2p63s3p64s
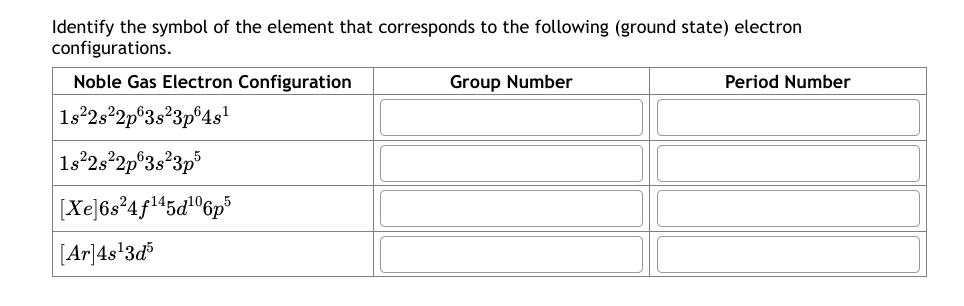
Identify the symbol of the element that corresponds to the following (ground state) electron configurations. Noble Gas Electron Configuration Group Number Period Number 1s2s2p63s3p64s 1s2s22p63s23p5 | [Xe]6s?4f145d106p [Ar] 4s3d5
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Noble Gas Electron Configuration The noble gas electron configuration is a shorthand notation used t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles The Quest For Insight
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
7th Edition
1464183953, 9781464183959
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



