Answered step by step
Verified Expert Solution
Question
1 Approved Answer
If 25mL of a 3MNaOH solution (pH=14) is mixed with 25mL of DCM containing aspirin, and the resultant solution is allowed settle which of the
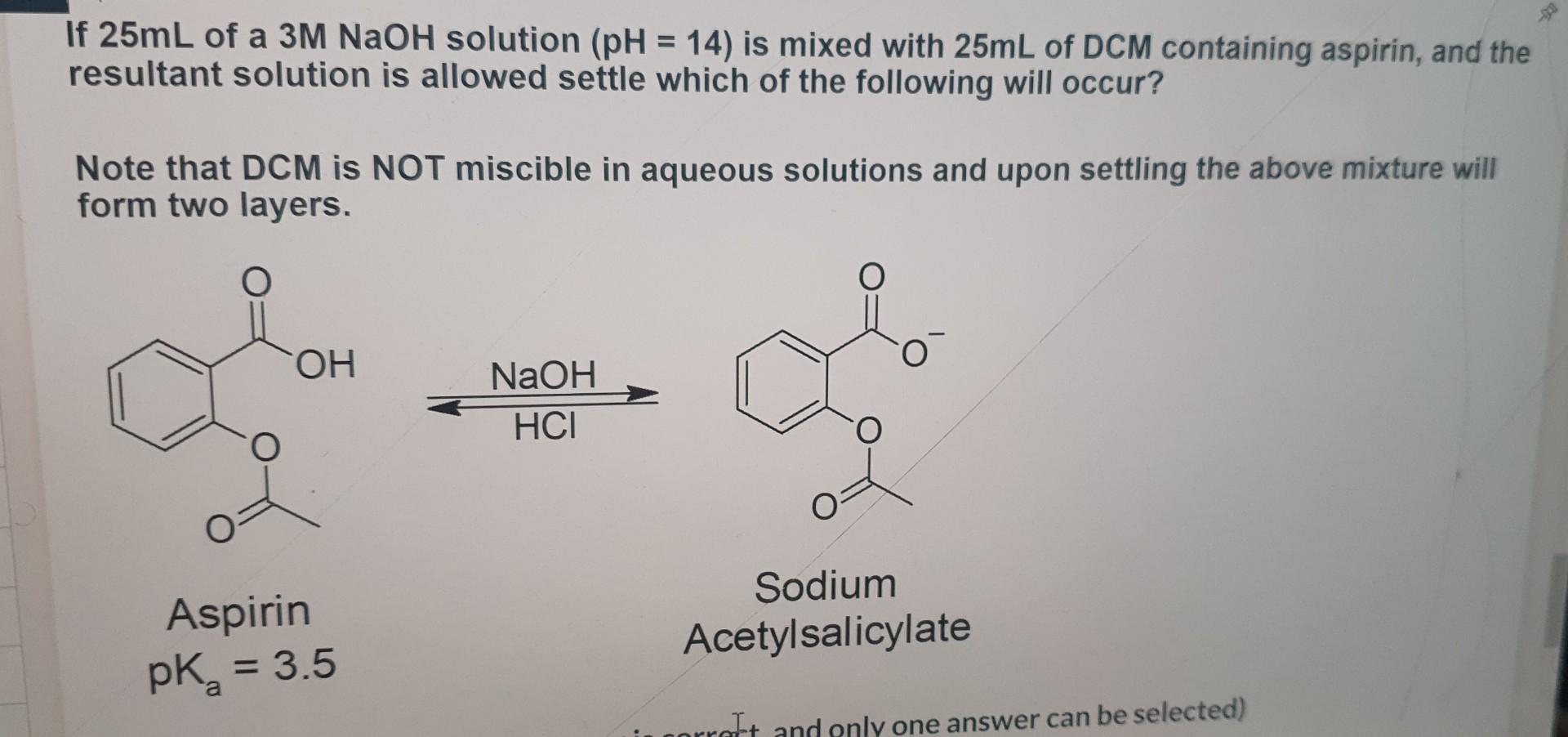

If 25mL of a 3MNaOH solution (pH=14) is mixed with 25mL of DCM containing aspirin, and the resultant solution is allowed settle which of the following will occur? Note that DCM is NOT miscible in aqueous solutions and upon settling the above mixture will form two layers. HClNaOH Aspirin Sodium pKa=3.5 Acetylsalicylate (Multiple Choice: Only one answer is correct, and only one answer can be selected) The vast majority of aspirin molecules will be converted to sodium acetylsalicylate and migrate to the aqueous layer ( NaOH). The vast majority of aspirin molecules will be converted to sodium acetylsalicylate and remain in the organic layer (DCM). The vast majority of aspirin molecules will be converted to sodium acetylsalicylate and precipitate/crash out of solution
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


