Question
Electroneutrality: Ecations= Zanions= % difference = Ecations - Eanions (Ecations + Zanions) 100 = Does this water sample exhibit electroneutrality? If not, what could
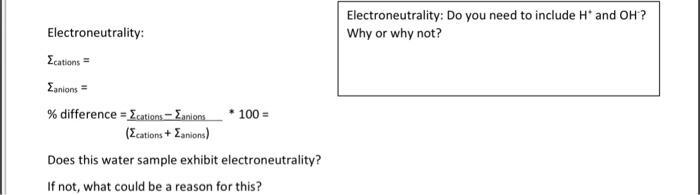
Electroneutrality: Ecations= Zanions= % difference = Ecations - Eanions (Ecations + Zanions) 100 = Does this water sample exhibit electroneutrality? If not, what could be a reason for this? Electroneutrality: Do you need to include H* and OH? Why or why not?
Step by Step Solution
3.41 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
Ques 4 a 2 b 1 44 200 1 sulfate ion NaSO s sodium lous are found in soluting ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Principles Of Managerial Finance
Authors: Lawrence J. Gitman, Chad J. Zutter
13th Edition
9780132738729, 136119468, 132738724, 978-0136119463
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



