Answered step by step
Verified Expert Solution
Question
1 Approved Answer
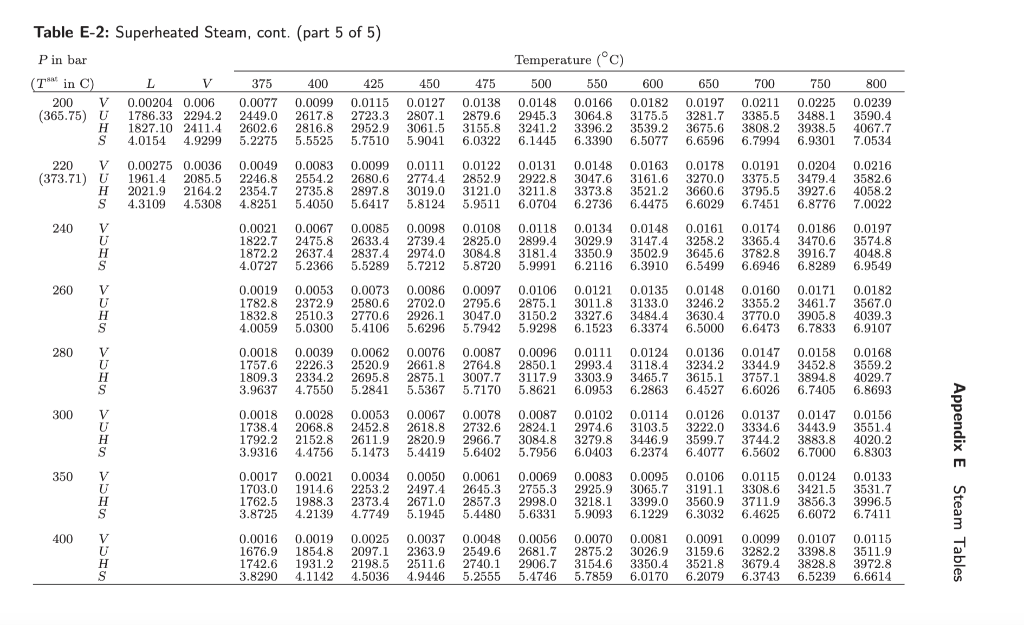
I'm confused how to answer problem 1g. Attached is the problem and the steam table, im just confused where to find the boiling point as
I'm confused how to answer problem 1g. Attached is the problem and the steam table, im just confused where to find the boiling point as it stops after 220 bar (373.71 degrees celsius) where the critical point is found.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


